Virology
Introduction
DNA Viruses
RNA Viruses
Introduction
● Virus → infectious agent composed of a genome of nucleic acid surrounded by a protein coat
● Obligate intracellular parasites that require an appropriate host cell for replication
● Viruses do not possess ribosomes
● not susceptible to commonly used antimicrobials used to treat other infectious diseases
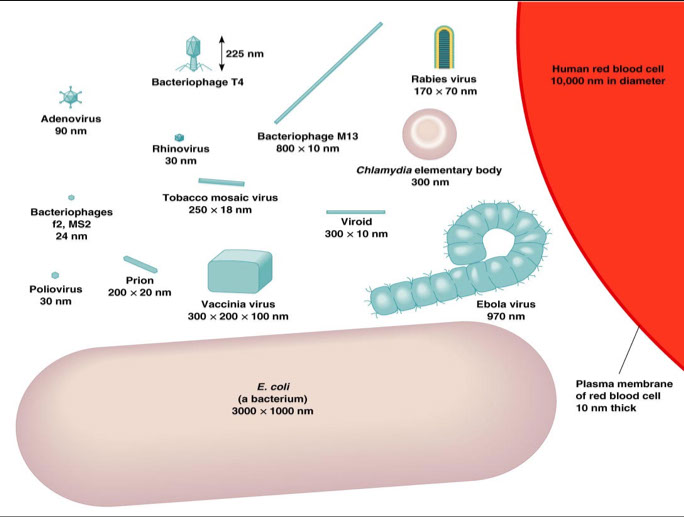
Small size → range from 20 to 1000 nm in length
● determined with the aid of electron microscopy
● Virion → complete, fully developed, viral particle
Biochemical Constituents of Viruses
Proteins
● Protein is MC chemical present in viruses
● Most protein is found in the protein coat (capsid) that surrounds and protects the nucleic acid core
● Some contain protein enzymes not provided by the host cell
Nucleic Acid
● Genetic information is contained in the nucleic acid
● Viruses contain only one basic type of nucleic acid, either DNA or RNA but not both
● The classification of viruses is primarily based on the type of nucleic acid present
● nucleic acids for various viral groups may be single-stranded, double-stranded, circular, or segmented
Lipids and Carbohydrates
● Certain viruses contain an envelope surrounding the nucleoprotein core
● The envelope consists of a lipid bilayer acquired from the host cell in which it replicated
● envelope normally susceptible to action of ether
● Envelopes may or may not be covered by spikes
● Spikes are carbohydrate-protein complexes that project from the surface of the envelope
● Lipids and carbohydrates are involved in attachment of the virus to the host cell
Types of Symmetry
● Viruses can be classified into several categories based on their general structural characteristics
Helical Symmetry
● Observed only in some RNA viruses
● either rod-shaped or coiled
● capsids are repeated units of a single polypeptide
Cubic Symmetry
● capsid consists of several different polypeptides grouped into subassemblies called capsomeres
● capsomeres are arranged into a regular polyhedron called an icosahedron
● An icosahedron consists of 20 faces, each an equilateral triangle, and 12 vertices
● The icosahedral capsid is a rigid structure enclosing the nucleic acid core
Complex Symmetry
● A miscellaneous category of viral structure that is neither helical or cubic
- bacteriophages have complex symmetry
Growth of Viruses
Growth of Animal Viruses
● Viruses can be grown in animals
● Some are propogated in embryonated hen’s eggs
● Many grown in appropriate cell or tissue cultures
Factors Influencing growth of animal viruses
● The type of animal (species)
● The age of the animal
● The route of inoculation
Embryonated Hen’s Eggs
● The age of the embryo
● The route of inoculation
● The younger the embryo, the larger the yolk sac
Cell or Tissue Culture
- MC method to dx most viruses (though fairly slow turnaround [?]), 3 general types of cells are used:
1. Primary cells - derived from tissue slices, die out fast, ie primary monkey kidney (PMK)
2. Continuous or established cell lines - HEp-2 from laryngeal ca, HeLa from cervical ca - transformed cells that can last >70-100 or indefinite generations
3. Diploid cell lines - from human embryos, last >50-70/100 generations
- EBV, arboviruses, + rubella not cultured, serum tests used
- cultures vary on ability to get cultured depending on virus, need to grow c different media (PMK, Hep2, HDF)
- MCC culture media contamination is Mycoplasma or Simian viruses
-- Simian virus cause false + hemadsorbtion (check controls)
The Viral Replication Cycle
● Viral replication normally occurs in a step-wise fashion
Adsorption
● initial attachment of a virus particle to a host cell
● Attachment involves a specific interaction between
structures on virus itself and receptor molecules on the surface of the appropriate host cell
Penetration
● The passage of the virus from the cell surface, across the cell membrane, and into the cytoplasm
● Penetration by two basic mechanisms: receptor-mediated endocytosis and membrane fusion
Uncoating
● Refers to stepwise process of disassembly of virion and enabling the expression of viral gene
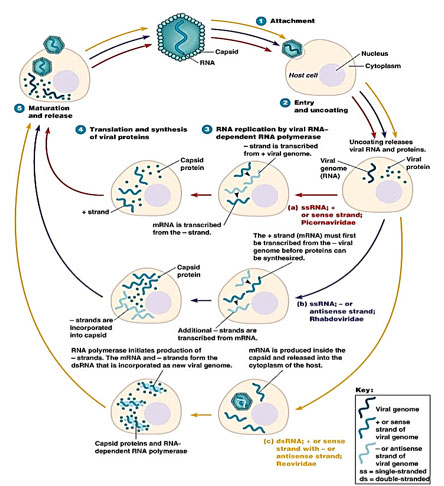
Synthesis
● Also referred to as the “eclipse phase”
● Nucleic acid and protein synthesis proceed independently
● Mechanisms vary depending on type of nucleic acid
● Mechanisms of RNA virus gene replication vary greatly as
far as specifics are concerned
Maturation
● Involves the assembly of nucleocapsids
● Assembly of RNA virus occurs in cytoplasm of the host cell and in the nucleus for DNA viruses
● Complete virus particles begin to appear
Liberation and Release of Progeny Viruses
● Depends on the type of virus and host cell
● May occur gradually as viruses are formed (budding) or more suddenly when the cell lyses
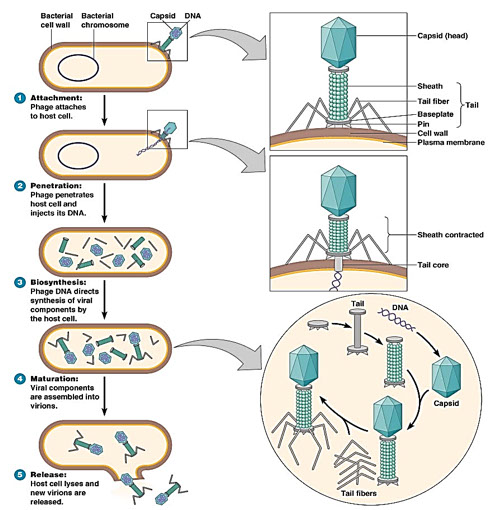
Multiplication of Bacteriophages
● Bacteriophage = viruses that replicate in bacteria
● multiply by two alternative mechanisms (lytic or lysogenic)
● The bacteriophage replication cycle is representive of viral replication in general
The lytic cycle
● Observed in the T-even bacteriophages
● Results in lysis and death of the host bacterium in replication cycle similar to that of animal viruses
The lysogenic cycle
● Do not cause lysis and death of the host cell when they multiply
● Lysogenic phages are also referred to as the temperate phages
● In lysogeny, the phage remains latent or inactive
● The phage genome is incorporated into the host cell’s genome as a prophage
● Results of lysogeny:
● lysogenic cells are immune to reinfection by the same phage
● phage conversion may occur
● allows for specialized transduction
Laboratory Diagnosis of Viral Infections
Isolation and Identification
1. Appropriate Specimens
● Throat washings ● Sputum
● Nasal swabs ● Spinal fluid
● Rectal swabs ● Serum
2. Neutralization tests and monoclonal Ab’s
3. Isolation of Viruses in Culture
● Cell culture is the cultivation in vitro of dissociated
single cells
● Viruses must be grown in appropriate host cells
● Types of cell cultures: Primary cell cultures, Cell lines, Established cell lines
● Virus-infected cultured cells exhibit morphological
changes that can be detected, analyzed, and graded
microscopically (Cytopathic Effects)
- phase contrast microscope used to detect viral cytopathic effect (CPE), use clues such as shape of CPE, cell type c CPE, time of detection to emergence of CPE (RSV fast, VZV, CMV, and RSV >2 wks)
-- no CPE c influenza or parainfluenza, but adsorb guinea pig RBCs on cell culture surface c hemagglutinins
Traditional Viral Culture
• Monolayer of tissue culture investing the inside of the tubes
• Look at tubes using an inverted microscope
• Detect Early Cytopathic effect: rounding of tissue
culture cells
• CPE under Phase Microscopy
• Mature CPE: a plaque (focus of dead and dying cells)
3 types of cell cultures in the market for viral isolation; frequently used in combination
1) Primary cell lines:
• Derived from actual tissues from animal organs,
usu. adult
• Kidneys, most common organ
• Limited passage viability
• Best system- support the widest range of viruses
• More expensive, harder to maintain a reliable
supply
2) Diploid or Semi-continuous cell lines:
• Derived from embryonic tissues
• Have the capacity for greater # sub-passages
before death (20-50)
• Exs. MRC-5: human embryonic pulmonary
fibroblasts
3) Heteroploid or Continuous cell lines:
• Neoplastic cells loose contact inhibition
• Indefinite # sub-passages; easier to handle
• Range of viruses supported is often limited
• HeLa, Vero, HEp-2, A549, LLC-MK2, etc.
Several disadvantages of traditional cell culture
• Delay in diagnosis for slow-growing viruses (1-5
weeks) (Ex., CMV)
• Low sensitivity in specimens in poor condition or
old (low virus viability) (Ex., VZV, RSV)
• Many viruses will not grow in cell culture (ie.,
hepatitis B, agents of viral gastroenteritis, parvovirus, papillomavirus, etc.)
c. Selected examples of viral cytopathic effects in tissue culture monolayers
• CMV: “focal” rounding of cells followed by formation of discrete plaques, whereas HSV is confluent, widespread, fast
• Parainfluenza Virus, Measles, RSV & the Paramyxoviridae: formation of syncytia
• Adenovirus: „bunch or grapes‟
Shell vial technique used c respiratory virus panels, incubated <3 days and stained c tagged fluorescein-labelled mono- or polyclonal antibodies detect presence of viral protein antigens in the monolayer cells; also for Herpes simplex, CMV, VZV, influenza, etc.
-- CMV gives apple-green intranuclear fluorescence
- with pulmonary specimens, shell vials stained first with Respiratory Virus Panel screening antibody pools ("cocktails")
- shell vial techniques are good bc rapid (though not immediate, available results in 1-4 days), high sensitivity 2/2 in vitro amplification of viral protein ags and specificity
Serology detects circulating ab's, current and past,
- optimal to get another serum sample to confirm IgG (inc 4x) after 10 days (IgM detected up to 4 mo s/p infx; IgG from 1 wk from start of infx, detectable for life)
Detection of antibodies to viruses in a patient‟s
serum.
- Screening Serological Assays are manufactured to be very "sensitive‟ (ie., few false negatives)
• Ex.: ELISA test for HIV antibody
• Microplate ELISA for HIV antibody: colored wells
indicate reactivity
• Confirmatory Serological Assays are manufactured to be very „specific‟ (ie., few false positives)
• Ex.: Western Blot test for HIV antibody
a. HIV-1 Western Blot
• Lane1: Positive Control
• Lane 2: Negative Control
• Sample A: Negative
• Sample B: Indeterminate
• Sample C: Positive (Two of the following three bands must be +: 24, 41, 120/160)
Signal Amplification Systems (amplify a signal used for detection, as in chemiluminescence or fluorescence, not target nucleic acid of organism). Examples:
• Branched DNA Signal Amplification System
(Chiron/Bayer)
• Hybrid Capture System (Digene Corporation)
Probe Amplification Systems (amplify a probe or the ligation product of two probes). Examples of the past: Ligase Chain Reaction (LCX, Abbott Corp)
Diagnosis by serology has several drawbacks
• Retrospective/indirect confirmation of infection.
• Immunocompromised patients may not
seroconvert
• If using paired sera (acute and convalescent),
patient may be unavailable for latter sample
• Large % of healthy/asymptomatic adults (as with
CMV) may show serologic evidence of past
infection. Careful with interpretation. Use
thresholds for positivity
• Some viruses (ie., HIV, rabies) produce clinical
disease months/years after seroconversion; mere
antibody presence may not correlate with
presence/absence of symptoms
- Mild local infections (such as genital HSV) may
produce no detectable humoral immune response
• False +‟s may occur when testing for IgM due to
rheumatoid factor, cross-reactions with connective
tissue diseases (ex,SLE)
• Patients given blood or blood products may give a
false positive result due to the transfer of antibody.
Measurement of Ab during the course of infection
1. Three basic techniques employed:
1) Complement Fixation, 2) Hemagglutination Inhibition, 3) Neutralization
2. Two serum samples required
● Acute → drawn as early as possible in the illness
● Convalescent → 10-14 days after acute sample
3. Interpretation of Results
● In two-fold dilution setups, a 4-fold or greater increase in Ab titer is diagnostic
● In a ten-fold dilution procedure, a 100-fold increase in Ab titer is diagnostic
● In both cases there will be a two-tube difference
between acute and convalescent samples
Rapid Diagnostic Techniques
1. Detection of Viral Genetic Material
a) DNA Probes
● Detection, Location, Quantitation
● No viral replication is necessary
● Dot blot procedures, FISH used to id HPV (gyn), CMV (resp), HSV (derm) and parvovirus B19 (BM)
b) Polymerase Chain Reaction (PCR)
● Amplification of material, direct DNA sequencing and branched (bDNA) amplification used to monitor pts c HIV and HCV
2. Immunofluorescence
a) Direct Immunofluorescent procedures - Direct Fluorescent Antibody (DFA) similar to FISH
- used for herpes and respiratory viruses
• Direct detection of antigens in smears or touch
preps from clinical specimens, by way of
fluorescence-labelled monoclonal antibodies (a rapid
technique).
• "In vitro‟ amplification does not take place.
• Positive immunofluorescence test for rabies virus
antigen, prepared from a brain touch prep from a rabid animal in a public health lab (Source: CDC)
-- Conclusions on DFAs:
• Speedy; Specific (uses monoclonals); May be insensitive (no amplification)
• But, can be very sensitive for labile viruses that
may die in transit (ex., VZV, RSV)
• Can be sensitive for antigens that are „pre-amplified
in-vivo‟ (ex,. CMV pp65 antigenemia assay, in
human lymphocytes)
b) Indirect Immunofluorescent procedures
3. Horseradish Peroxidase Conjugates (ELISA)
- Enzyme-linked Immunosobant Assay (EIA) used for GI and respiratory viruses
-- Enzyme Immunoassays (EIA) in Virology used
predominantly for two purposes:
1) Antigen detection, ie.: GI pathogens in stoolrota,
adeno, etc; respiratory tract pathogens- RSV, Influenza, etc
2) for Serology
• Most equipment used is either semi-automated
or automated
• Some is very versatile (ie, VIDAS)
4. Electron Microscopy
a) Observation of stained, thin, fixed sections of brain
and other tissues
b) Observation of stained various from vesicle fluid
c) Immunoelectron Microscopy
● Stool → Rotavirus, Hepatitis A, other enterics
● Brain biopsy → HSV
5. Molecular Amplification Diagnostic Methods
a) 3 general types of systems: Target, Signal,
and Probe Amplification Systems
1. Target Amplification Systems (amplify target
segment nucleic acid of the organism in the
clinical specimen; DNA or RNA)
- PCR is most common approach, by far.
Examples:
• Roche PCR systems (MWP, COBAS, LightCycler for real-time detection)
- Gen-Probe TMA (transcription-mediated amplification) systems
• NASBA TMA systems (Bio-Merieux, formerly by Organon Teknika)
• Strand Displacement Amplification systems (BD Probe Tec ET, Becton-Dickinson)
• Newer systems: multiplex PCR assays by various companies, run either closed-tube ("Real-time‟), or as open-tube PCR with Flow Cytometry-based detection on Luminex platforms.
-- The newest systems use "sample to answer” multiplex platforms with nucleic acid chips for detection. One example out of several platforms becoming available: FilmArray (Biofire)
2) FilmArray Respiratory Panel
a) Viral and Bacterial
Interferons
● Interferons are proteins made by virus-infected cells that protect other cells from viral infection
A. Interferons as anti-viral agents
● Human interferons are of three basic types that differ in antigenic, chemical, and biological properties
● IFN-α → derived from WBCs (leukocyte IFN)
● IFN-β → from diploid fibroblasts (fibroblast IFN)
● IFN-γ → from cells undergoing an immune reaction
B. Role in viral infections
● May expedite recovery and protective role in some virus infx bc interferons appear sooner than Ab’s
Classification of Viruses
Overall there are 20 important families of medically important human viruses, 6 DNA and 14 RNA viruses
Viruses are classified on the basis of;
● Type of Nucleic Acid (DNA vs RNA)
● Type of Symmetry
● Presence or absence of an envelope
● Type of host cell (host range)
● Geographic location
● How the virus is transmitted
● Others: serologic reactions, amino acid sequences of viral proteins, etc.
DNA Viruses
6 overall; DNA animal viruses separated to 3 classes based on structural differences in their viral genomes
Class I. Double-Stranded Linear
● Adenovirus ● Herpesvirus ● Poxvirus
Class II. Double-Stranded Circular
● Papovavirus ● Hepadnavirus
Class III. Single-Stranded Linear
● Parvovirus
RNA Viruses
14 families important for humans; RNA animal viruses are separated into five classes based on the nature of the RNA in the virions and their relationship to viral mRNA
● Characteristics in classification include following;
● Genome constitution and polarity (the numbers of multiple genome pieces)
● Presence or absence of Transcriptase in virions
● Infectivity of RNA
● Type of messenger (plus strand)
● Primary gene product
● Examples of RNA viral families
● Class I. → Picornaviruses & Coronaviruses
● Class II. → Paramyxoviruses
● Class III. → Orthomyxoviruses
● Class IV. → Reoviruses
● Class V. → Retroviruses
Selected Antiviral Agents
● Amantadine → Influenza A
● Acyclovir / Valacyclovir→ HSV 1 and 2
- Cidofovir --> HSV 1 and 2, VZV
- Foscarnet --> HSV 1 and 2, VZV, EBV
● Ganciclovir → HSV1 and 2, VZV, EBV, CMV, HHV6/7/8
● Famcyclovir → VZV
- n-Docosanol - HSV1 and 2, also RSV
- Trifuridine --> HSV1 and 2
● Virazole (Ribavirin) → RSV
● Azidothymidine (AZT) → HIV
● Protease Inhibitors → HIV (used in combination with AZT)



Cubic symmetry
Helical symmetry

Complex symmetry







DNA Viruses
The DNA animal viruses separated to 3 classes based on structural differences in their viral genomes, with 6 important families overall
Class I. Double-Stranded Linear
1) Adenovirus; 2) Herpesvirus; 3) Poxvirus
Class II. Double-Stranded Circular
4) Papovavirus 5) Hepadnavirus
Class III. Single-Stranded Linear
6) Parvovirus
Class 1: DS-Linear DNA
1) ADENOVIRUS
ds DNA, non-enveloped, replicates cell nucleus
- > 47 serotypes, six sub-genera (A to F)
-- low serotypes (1-14) assoc c resp infx
-- 11 and 21 assoc c hemorrhagic cystitis
-- 40, 41, 2 and 31 assoc c childhood GEitis (diarrhea)
Sx: Pneumonia in immunocompromised patients and
military recruits;
• Acute gastroenteritis children (40,41)
• Pharyngitis, pharyngo-conjunctival fever
• Keratoconjunctivitis
• Hemorrhagic cystitis
• Cervicitis, urethritis
• Disseminated disease
• Protruding fibrils involved in adhesion
1) Pearls from the inclusion body:
See ciliocytophthoria (CCP) - terminal bar and cilia detach from bronchial / ciliated columnar cells
Histo: may see intranuclear (IN) inclusions; Early inclusions eosinophilic, finely granular, smaller and
herpes-like (Cowdry A); late inclusions deeply basophilic and larger, with nucleocytoplasmic blurring (“smudge cells”)
- Alveolar lining cells contain large, homogeneous,
very characteristic IN: “Smudge cells”; inclusions are large, deeply basophilic (blue) with NO halo
• Nucleo-cytoplasmic blurring, small amount of
peripheral cytoplasm left
• No cytomegalia
• No multinuclearity
• No smooth ground-glass appearance
EM: Very crystalline arrangement of virions
2) HERPESVIRIDAE
All herpes family viruses are enveloped ds DNA
viruses.
● Herpesviridae is a large group of DNA viruses found worldwide; probably MC human viruses due to their abundance and tendency to remain latent in the body for life
3 subfamilies:
- Alphaherpesviruses - HSV-1, HSV-2, VZV
• Betaherpesviruses - CMV, HHV-6, HHV-7
• Gammaherpesviruses - EBV, HHV-8
● Large icosahedral viruses c lipid envelope.
● contain complex dsDNA as well as viral DNA polymerase and thymidine kinase enzyme
● All herpesviruses can undergo an alternative infection cycle, entering a quiescent state (latency) from which they can subsequently be reactivated.
● Latency → no virus replication, no symptoms, persists for lifetime
● Reactivation → may occur spontaneously or may occur as a result of immunosuppression, injury, fever, UV radiation, physical or emotional stress
Herpes simplex virus 1 (HHV-1)
● found primarily in the upper body
● transmitted via respiratory droplets or direct contact with saliva
● causes oropharyngeal disease
● Gingivostomatitis – young children
● Pharyngitis & tonsillitis - adults
● Recurrent infections manifest as “cold sores” or “fever blisters”; clusters of vesicles at lip border
● Skin infx --> Whitlow’s infection & Eczema herpeticum
● Eye infections also occur
● Keratoconjunctivitis – may lead to blindness
● Herpes encephalitis – 15% of encephalopathies, very high mortality rate, also assoc c necorisis and hemorrhage in temporal lobe, causing aphasia, hallucinations, abnormal behavior
Micro: intranuclear inclusions; Early inclusions amphophilic with “ground glass” appearance; late inclusions eosinophilic, homogeneous (Cowdy A) and surrounded by clear halo, with marginated chromatin; multinucleated syncytia (giant cells) and “molding”
Dx: CSF- inc protein, lots of RBCs, pleocytosis
Tx → acycloguanosine (Acyclovir), cannot cure a latent infection but can minimize or prevent recurrences
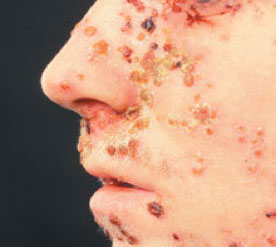
Herpes simplex virus 2 (HHV-2)
● in 1/5 of population, but only 2% of population suffers from genital herpes; in lower body
● HSV-1 and HSV-2 are closely related but can be distinguished serologically
● Transmission through sexual contact or during passage through the birth canal
Genital herpes - transmitted during birth (usually not transplacentally)
● Vesiculoulcerative lesions in the female vulva, cervix, and vagina and in the male penis
● Painful and highly contagious → possible fever, dysuria, lymphadenopathy
● lesions heal in about 10 days
● Complications → extragenital lesions, meningitis
● Neonatal herpes - Almost always symptomatic & symptoms are often severe
● Skin & mouth lesions, encephalitis (rarely), disseminated disease including CNS involvement
● Fatality rate may be high and survivors often have
permanent neurologic impairment
● Cesarean section recommended c active herpes
- rarely (not typically) causes encephalitis
Dx
cell culture definitive, DNA probes common too
-- Tzank smear made by smearing lesion on glass slide and stain c Giemsa, sens 7/10, follow negative tests c a culture
- serology is limited in HSV detection
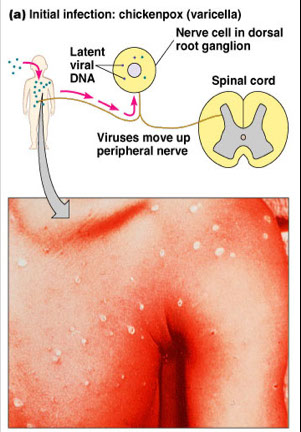
Varicella-Zoster Virus
● Very similar to HSV; only one antigenic type
● One virus causes both varicella and zoster
Sx
● Transmission → Inhalation, perhaps direct contact
● Infx almost always results in clinical manifestations, may be complicated by dangerous pneumonia in healthy adults and adolescents, esp pregs (can be congenitally passed transplacentally) and ICmp
- congenital infx depends on time of maternal infx
-- incidence highest in 3rd tri in utero, but very high in perinatal materal infx (>1/2, mortality 1/3, must admin VZIG if infx 5 days before to 2 days after birth)
Varicella (Chickenpox) - A mild childhood disease
● Incubation period → 14-21 days
● Fever, malaise, generalized rash
● Nonsynchronous pock development
● Itchy, but little or no pain
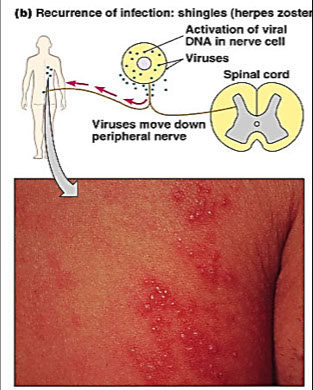
Zoster (Shingles) - recurrence in older adults
● Incubation period → 11 days ?
● Fever, malaise, unilateral rash
● Nonsynchronous pock development
● Intense pain over affected sensory nerves
- Ramsey-Hunt syndrome - otalgia, unilateral facial paresis, hearing loss, vertigo, tinnitus (rare in USA)
● Reyes Syndrome → encephalopathy & liver degeneration associated with VZV in children given aspirin
Micro: may produce intranuclear (IN) inclusions similar to herpes simplex
- similar to HSV, has the 3 M's: multinucleation, molded nuclei, marginated chromatin
Laboratory diagnosis usually not indicated
Treatment → Acyclovir
- Vaccine available
Cytomegalovirus (human CMV)
-Salivary Gland Virus
Induces cell gigantism
● Extremely labile (only 1/108 virions is infectious)
● Numerous transmission routes → virus can be shed in urine and other secretions
● intrauterine infection (1%, perinatal infection(8-60%), Saliva, Venereal, Blood transfusions, Organ transplantation
● Remains latent in secretory glands and kidneys
Sx: Normal Hosts
● Most infections are subclinical in normal hosts
● CMV mononucleosis → generally mild with malaise, myalgia, fever, liver dysfunction, hepatosplenomegaly in young children, lymphocytosis
● Spontaneous or after blood transfusion
Immunocompromised Hosts
● Severe CMV mononucleosis
● May be due to primary infection or reactivation
● CMV pneumonia is a frequent complication
Congenital & Perinatal Infections
- MC congenital infx in USA, transplacental,
● Cytomegalic Inclusion Disease → low birth weight, microcephaly, intracerebral calcs, HSmeg, jaundice, TBCpen, purpura; seen in 1/3 pregnancies c primary CMV, only 1/10 of those are severe
● Severe CNS involvement
● CMV is a leading cause of mental retardation
● Also → hearing loss, ocular abnormalities, etc.
- may cause chronic rejection in organ transplants
● Fatality rate close to 30%
Micro: may have intranuclear (IN) and intracytoplasmic (IC) inclusions; Cytomegaly; nucleolus often retained; single, amphophilic intranuclear inclusions (Cowdry A) inclusion. IC inclusions multiple, smaller, basophilic, GMS- and PAS-positive; IN inclusion formed early, IC inclusions later
- Large ("megalic‟) blood-vessel lining cell ("endothelial‟) ballooning into the lumen
- Intranuclear "Owl‟s-eye‟ inclusion with a halo
- Abundant pink (eosinophilic) intracytoplasmic
inclusions
- Ballooning renal tubular cells
Dx Serology (IgM + or 4x inc IgG), DFA, PCR, histology, culture
Epstein-Barr Virus (Infectious Mononucleosis)
● Transmission → respiratory secretions (saliva), rarely transfusions or transplants
● Referred to as “Kissing Disease”
● Infx epithelial cells of pharynx and salivary glands
● Infects B-lymphocytes via C3d r (CD21), CD8+ T-cells then react in PB, c atypical cells
● Transforms and “immortalizes” B cells which then express EBV antigens
Sx
Usually asx
● Immortalized cells secrete Ab (polyclonal activation)
● Remains latent in B cells
Infectious Mononucleosis - primarily a disease of young adults
● Incubation period → 30-50 days
● Symptoms → headache, malaise, fatigue, sore throat, pharyngeal inflammation, enlarged lymph nodes and spleen, occasional rash, inc LFTs (1/2)
● Atypical T lymphocytes in the blood
● Complications are rare but may include splenic rupture, thrombocytopenia, hemolytic anemia, encephalitis, meningitis
● Fatalities rare
Pharyngitis and Tonsillitis - in children 5-15 yo
● Generally mild and self-limited
Chronic EBV Infections - uncommon
● Severe, prolonged disease
● May be an association with “chronic fatigue syndrome” but strong evidence is lacking
Burkitt’s Lymphoma
Nasopharyngeal Carcinoma
Lymphoproliferative diseases (immunodeficient hosts)
Dx
Hoaglang criteria - inc WBCs c >1/2 lymph and >1/10 atypical lymphs, fever, pharyngitis, adenopathy, serology +
- EBV induces production of anti-i, rheumatoid factor, ANA and Paul-Bunnell heterophile abs (IgM, affinity for sheep / horse RBCs, spec but not sens)
-- heterophile ab + as Forsmann ab, also HIV, lupus or lymphoma
- has Monospot test uses rapid agglutination of beef and guinea pig kidney ags
- EBV serology sens and spec, uses IgG/IgM anti-viral capsid ag (anti-VCA)
- ISH c EBV-encoded RNA (EBER) done histologicaly
-- EBER localized to RS cells in Hodgkins and other EBV tumors
-- can also do ISH c BamHIW sequence of EBV DNA
Human Herpesvirus-6 (HHV-6)
- aka fourth dz, Duke dz, exanthem subitum
● Two recognized subtypes A and B
● Genome resembles CMV
● Transmission and site of latent infection is unknown
● Cause of Exanthem Subitum or Roseola Infantum
● Common infection in young children 6mo-3yrs.
● Symptoms → abrupt onset with high fever, rash on body but not on face
● Usually mild, but may convulse due to high fever
● Prompt recovery, no sequelae (possibly Chronic Fatigue Syndrome??), no treatment necessary
Human Herpesvirus-7 (HHV-7)
● Genome also resembles CMV
● Also implicated in cases of Exanthem Subitum (similar to HHV-6)
Human Herpesvirus-8 (HHV-8)
- seroprevalence low in general population, MC in male homosexuals
- etiologically assoc c all forms of Kaposi's sarcoma (an AIDS-related cancer c red-purple patches under skin and in internal organs)
- assoc c Primary Effusion Lymphoma and Multicentric Castleman's dz
Dx - FISH or PCR, or IHC (LANA-1 expression)
Herpes B virus (Cercopithecine herpesvirus 1)
- aka Monkey B virus
- can contaminate monkey kidneys, and rarely cause fatal neurologic dz in humans (>7/10 die if not tx'd properly)
3) POXVIRIDAE
● A family of large complex DNA viruses having no apparent symmetry
● Usually brick-shaped, 200-450nm, non-segmented dsDNA
Variola virus (variola major & variola minor)
● Transmitted by respiratory route; severe skin rash - Smallpox, eradicated in 1977
● Infects only humans
● Virus is found throughout the body
● Mortality rate → Variola major = 20%, Variola minor =1%
● Vaccination has resulted in eradication of the virus
● Can potentially be used as a biological weapon
Monkeypox
ds DNA enveloped virus of the Poxviridae family
● Orthopox viruses of animals such as cowpox and monkeypox can occasionally infect humans
● appeared among monkeys from Africa & East Asia
● outbreak of over 50 cases in the U.S. in 2003 in humans who had contact with pet prairie dogs
● No human to human transmission
● Symptoms closely resemble those of smallpox
Rare exanthematous disease of rain forest countries
of central and west Africa, due to Monkeypox virus
• 1958: first case in laboratory monkeys. Other hosts in Africa: African squirrels, rats, mice, rabbits. Natives
get it: death in 1-10% of humans. First reported in
Congo 1970, sporadic outbreaks.
• 6/2003 in Midwest: monkeypox amongst owners of
sick pet prairie dogs;contact with animal‟s blood,
body fluids, rash or from its bite; can spread person
to person via respiratory droplets
• Prairie dogs are native to North America, but had
been kept in close proximity to infected Gambian
rats imported from Ghana
Sx: like a mild smallpox, rash, fever, H/A, myalgias but with lymphadenopathy, which is not common with smallpox
• Prohibited: importation of all rodents from Africa
Labs: PCR, serology, EM, immunohistochemistry
• Smallpox vaccine: at least 85% effective in preventing monkeypox
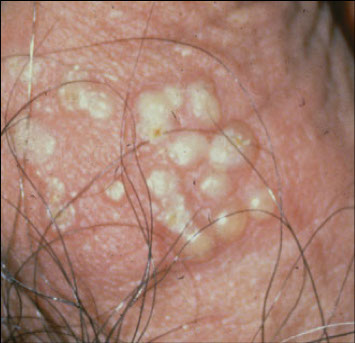
Molluscum Contagiosum Virus
● Causes a distinctive cutaneous wart-like lesion c waxy papules and central umbilication
- micro: prominent eosinophilic cytoplasmic inclusions
● Infects humans only
Class 2: DS-Circular DNA
4) PAPOVAVIRIDAE
Broken down into Polyomaviridae and Papillomaviridae
Polyomaviruses
JC and BK viruses, aq'd in infancy and latent in brain and urothelium
- JC virus assoc c Progressive Multifocal Leukoencephalopathy (PML)
- BK virus assoc c hemorrhagic cystitis and decoy cells in urine
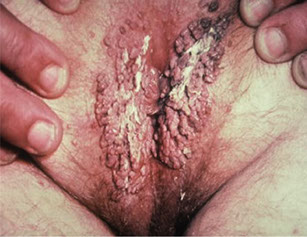
Papillomaviruses
- human papillomaviruses (HPV) cause common warts, sexually transmitted venereal warts
● tumors of the resp, intestinal, and genital tracts
- in b9 lesions HPV DNA is episomal, and in malignant lesions is integrated to host cell DNA
-- E6 and E7 genes assoc c oncogenesis
Sx
Epidermodysplasia verruciformis, recurrent respiratory papillomatosis (RRP), condyloma accuminata, Bowenoid papulosis, anogenital cancers
● More than 60 types have been identified
● HPV-2 & HPV-4 are associated with common warts
● Numerous types are associated with genital warts
● HPV 16 and 18 - moderate to strong association with cervical cancer

Herpes gingivostomatatitis

Eczema herpeticum-HSV1
Genital herpes - HSV2
Roseola infantum

Smallpox (Variola)

Molluscum contagiosum

Papillomavirus

Genital warts
5) HEPADNAVIRUS
Hepatitis B virus
- DNA hepadnavirus, only (double-stranded) DNA hepatitis virus, rest are RNA viruses, intact virion called a Dane particle
- has cellular RNA polymerase that transcribes RNA from DNA template, (reverse transcriptase transcrives DNA genome from RNA intermediate)
- chronic HBV assoc c PAN and glomerulonephritis from immune complex deposition
- 1/10 become chronic
- 1/2 of pts do not remember having acute infx
- 9/10 infants infected transplacentally and 1/10 IC, and 1/20 health ppl get chronic dz
Serology
HBsAg first to become + in acute infx (though HBV DNA appears before this)ghdf, E-ag assoc c amt of active viral replication, if HBsAg neg pt cannot transmit dz, E-ag also indicated possibility of transplacental spread, core Ag does not circulate
-- development of Ab's usually correlated c start of sx amd anti-HBe and HBs correlate c sx resolution
- chronic HBV = persistance of HBsAg after acute phase, if asx then called carrier state
- IgG HAVAb - protects against future infx, indiccative of prior infx
- IgM HAVAb - best for detection of active hep A, is an IgM ab against HAV
- HBsAg - Ag on HBV surface; indicates active dz, pt in carrier state (chronic dz) if persists
- HBsAb - Ab to HBsAg that provides immunity to HBV; pt is recovered from exposure
- HBcAg - Ag assoc c HBV core, indicating new dz and active viral replication
- HBcAb - Ab to HBcAg, + in window period,
-- IgM HBcAb indicative of recent dz, + in window period, can persist up to 1 yr after acute infx and come up in flares
-- IgG HBcAb of chronic dz or resolved infx
- HBeAg - another Ag in the HBV core, indicative of active infx and transmissibility **HBE = Enfective **
-- C or pre-C gene mutations can cause bad forms of fulminant hepatitis c liver failure
- HBeAb - Ab to e Ag, indicative of low contagious, but does not imply resolved infx or immunity
Genes - pre-S encodes hepatocyte receptor-binding site; S gene the surface Ag, C gene the core ag and e ag, the P gene encodes DNA polymerase
Molecular assays used to dx dz, monitor tx, and distinguish replicating from non-replicating dz
- HBV DNA can be detected weeks b4 HBsAg inc, and may be better than HBe Ag to detect replication
-
Hepatitis D virus (HDV)
- delta agent
Defective virus that req's HBsAg as envelope
- can co-infect or superinfect c HBV (superinfx worse)
Hepatitis E virus (HEV)
- RNA herpesvirus, rare in USA
- transmitted enterically (Vowels to the bowels!), H20-born epidemics, similar to HAV, M>F, but high mortality in pregs (1/3); Dx: IgM anti-HEV
6) PARVOVIRUS
- ss linear DNA viruses
B19 virus
L. “parvo” = small
Genera (or subfamily): Erythrovirus
- B19 virus associated with erythema infectiosum and aplastic crisis of sickle cell anemia*
Human Bocavirus
Adeno-associated virus (AAV) 2
- new agent of lower respiratory tract infection
Genera: Dependovirus
- Defective viruses (infect humans in presence of a
helper adenovirus)
RNA Viruses
1. Picornaviridae
- means Pico (small) RNA virus; includes the enteroviruses, rhinoviruses, and Poliovirus groups (enteric stuff)
*** PERCH on a Pike (Pico) *** Polio, Echo, Rhino, Coxsackie, HAV ***
- RT-PCR is gold standard to dx enterovirus in CSF, but should do cell culture as well
Poliovirus
● Small, naked, icosahedral viruses
● Contain a ssRNA that is non-segmented
● Size → 22 – 30nm
● Three antigenic types
● 90-95% of infections are asymptomatic → No disease, no sequalae
● About 4% present with a minor illness (Abortive infection)
● Only 1% lead to nonparalytic polio and some lead to paralytic poliomyelitis
● Poliomyelitis is an acute illness which destroys the lower motor neurons of the spinal ventral horn and brainstem
● This results in flaccid, asymmetric weakness or paralysis
● Infection is acquired by ingestion of food or water
contaminated with the polio virus
Sx: Abortive poliomyelitis → headache, sore throat, nausea (non-specific cold-like symptoms)
● Nonparalytic poliomyelitis → symptoms consistent with aseptic meningitis
● Paralytic poliomyelitis → myalgia, asymmetric muscle weakness followed by muscle paralysis
● Respiratory paralysis may also occur
● Paralysis most often affects the lower limbs
● Postpoliomyelitis syndrome → 20-30% of those who recover from paralytic poliomyelitis experience a new onset of muscle weakness, pain, atrophy, and fatigue 25-35 yrs. after the acute illness
Tx: specific antiviral agents are not available
● Vaccination is the only effective means of preventing polio
● Live-attenuated vaccine → Sabin vaccine
● Killed vaccine → Salk vaccine (no adverse effects)
● Live vaccine may undergo reversion to a virulent form in the human intestinal tract
● Vaccine associated polio accounts for the few cases seen in the U.S. each year
● Wild-type polio infections continue to occur in countries with low immunization rates → subSaharan Africa and southern Asia
Echovirus
Can cause aseptic meningitis, comes form pools?
Rhinoviruses
● Most frequent cause of common-cold syndrome
● Replicate in the nasal passages
● Over 100 serotypes are known
● Transmission → respiratory droplets and hand-to-hand contact
Coxsackie A
Cause painful oral infx called hepangina and hand-foot-mouth dz
Coxsackie B
Causes epidemic pleurodynia (the grippe), myocarditis and pericarditist
Hepatitis A virus (HAV)
- RNA picornavirus
fecal-oral transmission (Vowels to the Bowels!), short incubation (3 wks) very common, no carriers, 1/20 may have recurrent dz in first few mo after infx
Dx
IgM anti-HAV in acute infx, IgG anti-HAV can be chronic or acute infx
2. Caliciviridae
L. "calix‟ chalice, cup, goblet; capsid has cup-shaped holes or depressions
Calicivirus
Vesicular exanthem virus (swine)
- Difficult or impossible to propagate in vitro
Human gastroenteritis, Norwalk virus, hepatitis E virus; NLV now: Norovirus
3. Astroviridae
L. "astro‟ = star
Human astrovirus 1, 8 serotypes
4. Togaviridae
L. "toga‟ = cloak or coat, 2/2 the tight viral envelope
- includes alphavirus, and rubivirus (rubella)
Sindbis virus, an Alphavirus
Group A arboviruses, eg, Eastern equine encephalitis virus
Chikunguya
Single-stranded, positive-sense RNA alphavirus (family of Togaviridae) transmitted primarily by Aedes aegytpii and albopictus
- these mosquitos can also transmit dendue virus
- causes high fever, severe arthralgia, arthritis, rash and lymphopenia
- Rubella virus causes fever c rash (German measels), most serious complications occur if aq'd in 1st trimester preg (deafness, glaucoma, micropthalmia, PDA), Smegaly, TBCpenia
5. Flaviviridae
L. "flavus‟ = yellow jaundice due to severe hepatitis in
yellow fever
Yellow Fever Virus
- Dengue and yellow fever - transmitted by Aedes aegyti or Aedes albopticus, causing fever, aches, jaundice, and hemorrhage if severe
-- Dengue hemorrhagic fever seen c reinfection
-- yellow fever can cause midzonal necrosis, Councilman bodies, microvesicular steatosis in liver
Hepatitis C virus (HCV)
- RNA flavivirus
transmitted by blood, resembles HBV in sx, can also have carriers, MC in IVDU and post-transplant
- up to 17/20 get chronic dz, 3/20 of these get cirrhosis, 1/20 of these get HCC (esp worrisome c lots of fibrosis [Ishak 3] on liver bx)
- C = Chronic, Carriers, Carcinoma, Cirrhosis
- pts also get mixed cryoglobulinemia, glomerulonephritis, aplastic anemia
Dx
EIA-assays for anti-HCV ab's, cannot distinguish acute from chronic on labs (no reason to do so?), most pts dx'd in chronic phase, can now do qualitative HCV RNA test
Tx
- Peg-a-INF and ribavirin
-- Sustained Viral Response (SVR) = negative HCV RNA for 6 mo after tx
- HCV genotyping can be done by molecular (direct sequencing or RLFP) or serologic assays based on type-specific Ab's
- liver bx important in assessment of HCV RNA genotype 1 or 4-9
Hepatitis G virus
- aka GBV-C
- RNA flavivirus
Similar to HCV, parenteral infx c chronic infx
West Nile virus
Basic Science Pearls
Flaviviridae family: ssRNA enveloped icosahedral viruses; also includes SLE, Yellow Fever, Dengue fever, Japanese Encephalitis
1937: first isolated febrile woman living West Nile
Province of Uganda
- Commonly found in Africa, West Asia, Middle
East, not USA until 1999
• Several epidemics 1950-2000 various countries. Not
commonly assoc. with CNS involvement
• 1999: NYC, first human cases: cluster 8 patients
with encephalitis
• Coincidentally: avian pathologist had recently
reported increased # dead crows, flamingoes,
herons, bald eagles at Bronx Zoo, Queens Zoo
• Mosquito-borne: > 40 species as vectors, mostly
Culex spp, esp Culex pipiens
• In mosquito salivary gland: upon taking blood meal,
injects virus
• Isolated from numerous wild and migratory
birds: amplifier hosts
• Can also infect humans, horses, other mammals
Clinical Medicine Pearls
• Most infections are asymptomatic
• 20%: West Nile Fever: fever, malaise, N/V, H/A,
myalgia/rash/lymphadenop.
• Severe infection: 1 in 150, esp. with advanced
age; encephalitis, meningitis, meningoencephalitis.
Tx: supportive, no specific antiviral; ribavirin + alpha-2b interferon has some activity
Public Health measures: surveillance in birds and
mosquitoes; aerial spraying; serologic population
surveys; sentinel chickens
• Avoid mosquitoes; insect repellent (DEET)
Laboratory Medicine Pearls
• Serology, local or state health departments, in CSF
and serum: MAC-ELISA = IgM antibody-capture
enzyme-linked immunoassay
• Serologic x-react may occur with members of
Japanese encephalitis serocomplex, esp. SLE. For
test, antigens from both WNV and SLE virus are
used.
• If + to both: use PRNT (plaque reduction
neutralization test) with separate tube cultures of
Vero cells (African green monkey kidney) each
inoculated with one of the two viruses, for
specificity
• Tissues: RT-PCR with sequencing; immunohistochemistry
Zika virus
Arthropod (mosquito)-born RNA flavivirus; 1/5 infected get sx c fever, rash, arthralgia, conjunctivitis
- neurologic dz (2/2 neutrotropism of virus): congenital microcephaly, Guillain-Barre, myelitis, meningoenceohalitis
- named after Ugandan forest where first isolated in monkeys in 1947, first human cases found in 1952
- RNA detectable in lots of body fluids
- low fatality, up to 2 wk incubation
Dx: rRT-PCR (may cross-react c other flaviviruses)
Tx: non-specific / symptomatic / prevention
6: Reoviridae
Reovirus 1, 2, and 3
Orbivirus
Bluetongue virus, Colorado tick fever virus
Rotavirus
Rotavirus groups A, B, C, and G (MCC viral GE-itis)
7. Orthomyxoviridae
L. "ortho‟ = true; "myxein‟ = mucus.
- Affinity for respiratory mucous membranes
Lipid envelope c surface proteins, MC is hemagglutinin, which binds sialic-acid-containing receptos on resp epithelial cells and is expressed on their cell surface (basis of hemadsorption test, + in paraflu and influenza A + B)
Influenza Viruses
● Myxoviruses have an affinity for mucins (aid in attachment)
● Single-stranded, segmented RNA viruses with helical symmetry; 100nm, roughly spherical but irregular or pleomorphic
● Genetic Reassortment → segmented genomes from related viruses may result in mixtures of parental genomes in the progeny virions
● Three immunological types: A, B, and C, determined by the nucleoprotein antigen in the viral core
● Influenza A viruses are divided into subtypes based on two
proteins on the surface of the virus
● Hemagglutinin (16 subtypes, attachment functions - H spikes)
● Neuraminidase (9 subtypes, release of virions from cell - N spikes)
● Antigenic Drift → Minor antigenic changes in H and N subtypes
● Antigenic Shift → Major, sudden antigenic changes
● Lipid envelope derived from the host cell membrane (sensitive to ether)
Transmission
● Influenza A viruses are found in many different animals (ducks, chickens, pigs, whales, horses, seals, etc.
● Respiratory Route → Aerosol droplet spread from person to person person (rarely animal to person)
● Can survive for short periods on surfaces
● Virus concentration is high in nasal & tracheal secretions
Incidence and Persons At Risk
● During winter influenza epidemics, 10-20% of people become infected
● Estimated 36,000 deaths and 114,000 hospitalizations per year in the U.S. due to influenza
● At risk individuals → Very young, elderly, debilitated, and pregnant women
Clinical Course
● Short incubation time → 1-2 days
● Respiratory epithelial cells become infected
● IFN produced in one day, Ab’s in 1-2wks.
● Symptoms → chills, headache, dry cough, high fever, muscle aches, malaise, anorexia
● Pneumonia may develop resulting in “excess deaths”
● Caused by viral or secondary bacterial infections
● Factors → loss of ciliary clearance, phagocytic cell
dysfunction, alveolar exudate
● Organisms involved → Staph aureus, Strep pneumoniae, Haemophilus influenzae
● Fatality rates up to 42% with Staph aureus
● Reye’s Syndrome may develop when aspirin is used to control fever in children (<15 yo) with influenza, is an enceohalopathy c nausea and vomiting c high mortality
Inlfuenza A > Influenza B, cannot develop immunity 2/2 antigenic changes, esp prevalent in cold months
- Influenza A sx worse than B
- may get complicated by bacterial pneumonia (MCC death), Reye syndrome, myositis, myocarditis, Guillain-Barre
Micro: nonspecific; necrotizing bronchitis and tracheitis, DAD, thrombi and hemorrhage, edema
- inclusions are not produced
Control / Treatment
● Influenza types A or B cause epidemics of disease almost every winter season
● Influenza type C causes a mild respiratory illness but no epidemics
● Laboratory diagnosis is important at the beginning of the season or epidemic
● Once identified, clinical presentation is sufficient for diagnosis
● Amantidine (not effective c type B) or Rimantidine are effective prior to contact with virus
- oseltamivir (Tamiflu, neuraminidase inhib) and zanamivir (Relenza) effective against resistant strains
● Vaccines → Killed viruses, each year’s vaccine includes Ag to types A and B, but not type C (flu shots)
Dx
Culture is gold standard, on nasopharyngeal secretion, sputum or sqabs (shell-vial technique can give dx in 2-3 days)
- DFA has high sens; rtPCR can detect viral RNA, serology can be done retrospectively
H5N1 Avian Influenza A Virus
• Many bird flu virus sub-types (15), some very
contagious and very dangerous for domesticated
birds: HPAI (highly pathogenic), includes H5N1
strain, culprit current outbreak in poultry in Asia
since late 2003. Usu do not infect humans, thus little
or no natural immune protection exists
• Human flu A viruses (H1N1, H3N2, H1N2, H2N2)
• 1997, Hong Kong: first human case of H5N1, from
infected poultry. As of 3/11/2009: 431 cases/265
deaths in humans (mortality is 65%); Vietnam,
Thailand, Indonesia, Cambodia; now found in birds
Turkey, Romania, a Greek isle, and other sites, from
infected migratory birds; also present pigs, housecats, tigers, leopards
• Late May 2006: Indonesia, 7-member family; all 7
became ill will H5N1, 6 of 7 died; only 1 member
known to have contact with chickens. First evidence
of cluster of human-to-human transmission within a
group living together
• Thus: some evidence exists isolated cases of human-to-human spread, but spread has not continued beyond one person or outside of that group cluster. If and when virus rearranges to achieve “efficient and sustained transmission in humans”: risk for potential pandemic spread world-wide
• H5N1 virus in Asia resistant to amantadine and
rimantadine. Two new drugs Tamiflu (oseltamavir) and Relenza (zanamavir): anecdotal reports R to Tamiflu
• Vaccine development: enormous efforts FDA/NIH
led to approval of 1st US vaccine April 2007,
manufactured by Sanofi-Aventis. Interim measure
until more immunogenic ones are developed,
requires higher antigen dose than seasonal flu
vaccines and 2 shots within 28 days apart
Labs: we are not to culture amplify in hospital BSL-2 labs; RT-PCR primers/probes for H5 (N1) distributed state health labs, to "sentinel‟ labs (part of LRN); send to CDC
8. Paramyxoviridae
L. "para‟ = related to, or resembling, the orthomyxoviridae
Parainfluenza virus
Paramyxovirus types 1 and 2 cause croup in kiddoes 2-6 yo, type 3 causes respiratory bronchiolitis (2nd MCC, after RSV) in infants <2 yo
- can have intracytoplasmic (IC) inclusions; Multinucleated syncytia (giant cells); IC inclusions, when present, pleomorphic, eosinophilic and indistinct
Mumps virus
Rubulavirus, A single antigenic type
● Humans are natural host
● Transmission via aerosol droplets or direct inoculation
● Replication occurs in nasal or respiratory tract epithelial cells
● Viremia to salivary glands
● Secondary targets → testes and ovaries
● Damage is due to epithelial cell destruction
Sx: 1/3 cases are subclinical
● Prodrome period (malaise/anorexia) is followed by rapid enlargement of parotid (parotiditis) and salivary glands (single or bilateral)
● Orchitis may develop when testes are involved
● Aseptic meningitis → 15% of all post-infectious
encephalitis cases are caused by the mumps virus
● Dramatic decrease in # of infections due to live,
attenuated vaccine
Immunity / Diagnosis
● Permanent immunity after single infection
● Presence of Ab correlates with protection
● Excellent vaccine available, introduced in 1967
● Virus is difficult to isolate
● Diagnosis based on clinical presentation
Measles (Rubeola)
Morbillivirus, Single antigenic type
● Humans are the natural host
● Transmission through droplet aerosols
● Virus replicates in RES system
● Secondary viremia seeds epithelial cells
● Rash → produced by complexes of viral Ag & Ab
Sx: Typical Measles - prodromal fever, sneezing & other cold symptoms, redness of eyes
● Koplik’s Spots → seen in 85% of cases
● Rash
- Atypical Measles - Measles without rash or unusual rash
● Received killed measles vaccine as children
● Killed vaccine resulted in incomplete protection
- IC pts may get progressive fatal measels giant cell pneumonia (Warthin-Finkledy giant cells have nuclear and cytoplasmic inclusions, MNGCs)
Complications - MC complication is otitis media; also pneumonia & encephalitis, appendicitis from mesenteric adenitis or direct measels involvement of appendix;
- rarely chronic encephalitis known as subacute sclerosing panencephalitis (SSPE)
Micro: interstitial pneumonia c mononuclear infiltrate, DAD, MNGC c nuclear and cytoplasmic inclusions (Warthin-Fincklety cells, and Cowdry A/B)
- Cowdry type B is an inclusion w/o chromatin margination
- can have intranuclear (IN) and intracytoplasmic (IC) inclusions; Multinucleated syncytia (giant cells); IN inclusions eosinophilic and herpes-like; IC inclusions pleomorphic, deeply eosinophilic, hyalinized, tallow-like
Immunity / Diagnosis - Infection or live vaccine gives lifelong immunity
● Vaccine has resulted in dramatic decrease in # of cases
● Diagnosis based on clinical grounds
Human Respiratory Syncytial Virus
(Pneumovirus)
MCC LRTI (pneumonia and respiratory bronchiolitis)
-2 distinct serologic groups (1 in most epidemics)
● Most important cause of lower respiratory tract illness in infants and young children
● Causes about 4500 deaths per year
● May also cause infection in adults
● Large droplet aerosol transmission → person to person
● Virus replicates in epithelial cells of the nasopharynx and is carried by secretions into the lower respiratory tract
● CMI important in clearing infection
Sx: Most infections are asymptomatic
● Incubation period about 4 days
● Disease is concentrated in the winter months
● A major cause of nosocomial infection
● Can cause fever, rhinitis, sore throat, cough, croup
● Complications → bronchitis, bronchopneumonia, and interstitial pneumonia
Immunity - Severe RSV disease occurs in infants 2-4mo. of age as maternal Ab declines
● RSV is an inefficient producer of IFN
● Secretory IgA is important but not long lasting
- bronchiolitis uncommon after 12 mo age
Micro: can have intracytoplasmic (IC) inclusions; Multinucleated syncytia (giant cells); multiple, discrete, smoothly contoured and deeply eosinophilic IC inclusions
Tx/ Dx
● Isolation of virus or demonstration of viral Ag in tissue
● Demonstration of viral Ag in respiratory secretions by DFA is the most common method of diagnosis
● Also → EIA forming syncytia in Hep2 cells
● Ribavirin → administered in an aerosol
Human Metapneumovirus
Newly identified member family Paramyxoviridae,
closely related to RSV
First isolated in Netherlands: hospitalized children
with acute community-acquired respiratory tract
involvement, with negative tests for RSV, flu,
paraflu, adeno
• May progress to flu-like sx, bronchiolitis,
pneumonitis
9. Rhabdoviridae
- Only a single virus in this family affects humans
Rabies virus
- Single-stranded, non-segmented RNA virus
● Helical symmetry, enveloped
Latin "rhabdo" = Bullet-Shaped, 50-95nm x 130-389 nm
Found in a wide variety of animals → raccoons, skunks, squirrels, foxes, bats
● Domestic dogs and cats may serve as a reservoir
● Humans usually infected by the bite of an animal
● U.S. → human rabies cases are rare
● Most cases in U.S. are from a bat strain of virus
● Worldwide, especially rural areas of Africa & Asia
→ estimated 55,000 deaths/yr.
● Virus replicates locally at site of the bite wound
● Then travels along peripheral nerves to the brain
● Virus then replicates in the gray matter (brain)
● From the brain, the virus travels along autonomic nerves to lungs, kidney, adrenal medulla, and salivary glands
● Incubation period → 0ne to eight weeks (sometimes longer – several months to several years)
Sx: Abnormal sensations at site of bite leading to a fatal encephalitis
● hallucinations, seizures, weakness, mental dysfunction, paralysis, coma, ultimately death
● Many patients show classic signs of “hydrophobia”
● Hydrophobia → infected individuals painful inability to swallow liquids, leading to avoidance
● Once symptoms begin, death is inevitable
Dx: rests on history of exposure and signs &
symptoms characteristic of rabies
● Diagnosis is made postmortem in 80% of cases
● Demonstration of Negri bodies (intracytoplasmic eosinophilic inclusion bodies) in Purkinje cells of the hippocampus and cerebellum, and Babes nodules
● FA staining and PCR
Tx: No effective treatment once the individual has clinical symptoms
● Preexposure prophylaxis indicated for at risk people
● Human diploid cell vaccine (HDCV)
● Postexposure prophylaxis → treatment instituted soon after bite from suspected rabid animals
● Includes passive immunization with antirabies Ig and and active immunization with the rabies vaccine (HDCV)
10. Bunyavirus
"Bunya" comes from Bunyamwera, a town in Uganda, Africa where is was first described
Includes hantavirus, California encephalitis, and hemorrhagic fever viruses
- hantavirus aq'd by rodent excrement aerosolized, that causes fatal acute resp failure in 1/2, looks like DAD
Hantavirus
ssRNA, icosahedral, enveloped member of Bunyaviridae family
• Hantavirus Pulmonary Syndrome (HPS): deadly
disease transmitted by infected rodents through
urine, droppings, saliva
• 1993: first cases identified in USA in the SW (fourcorner area); since, identified throughout the US
• Humans breathe in the aerosolized virus
• Fever, fatigue, myalgias, dyspnea progressing rapidly to respiratory failure
• Can not be transmitted from person-to-person in USA; can occur with hantaviral species in other parts of world
• Hantavirus or "Sin Nombre Virus‟:
• Hemorrhagic fever syndromes: Filo, Arena, Flavi
and Bunya families
• Reservoirs: deer mouse (Peromyscus maniculatus);
cotton and rice rats, white-footed mouse
Labs: serology positive for IgM or IgG; pos immunohistochemistry or RT-PCR from autopsy tissues; pos RT-PCR from blood
EM and micro: abundant levels of viral antigens in pulmonary microvasculature with almost no mature virions, little tissue CPE but an overwhelming immunologic reaction
Prevention: rodent control: SEAL UP rodent entry holes, TRAP UP rats with snap traps, CLEAN
UP rodent-infested areas and nesting sites with bleach
11. Coronavirus
Group of RNA viruses that look like corona or halo in EM which is an array of surface projections on viral envelope
- ssRNA is largest known RNA virus genome
- highest known freq of recombination of any positive stranded RNA virus
- causes major illness in animals and ~1/3 common colds in humans including SARS virus (2002)
SARS
Viral respiratory illness appearing first in Southern China in 11/02, recognized as global threat in 3/03; 1st new pandemic 21st century
• Agent: SARS-CoV, a previously unrecognized
coronavirus, a mutated agent
• ssRNA enveloped virus
• Coronaviruses cause 1/3 of human common colds,
also enteric infection.
• New strain possibly mutated in Chinese Horseshoe
Bat.
Clinical Medicine Pearls (for SARS):
- Suspected case: temp.>100.4F (38)C; cough or
breathing difficulty; close contact within 10d
patient with SARS or travel to SARS + location
- Probable case: above + pneumonia; or above +
pathology revealing DAD/ARDS
- Fever, chills, H/A, malaise, myalgias, diarrhea;
then respiratory phase: cough, dyspnea, hypoxia.
Majority are symptomatic.
• Hong Kong cohort: 50% were health-care workers;
15% on assisted-ventilation
- Spread by respiratory droplet aerosol; and contact
with infected fomites
• Infection Control: N95 mask, negative pressure
rooms; surgical masks for outpatients
• World-wide 11/02-7/03: >8,000 cases; 800 deaths;
in USA: 8 cases with no deaths
• April 2004: lab-acquired cases in China
Laboratory Medicine Pearls (for SARS)
• Pulmonary postmortem findings: DAD/ARDS
• Clinical labs: RT-PCR in nasal secretions, serum,
stool at a LRN facility; confirm at a second facility
• Serology in state health dept, confirm at CDC
• Viral culture only in BSL-3 labs. Lab accidents
already occurred in Taiwan, Singapore, Beijing.
• In routine lab, any potential SARS + specimen: use
standard BSL-2 hood and universal precautions
Micro: DAD, hemorrhage, edema, multinucleated in 1/10, no viral inclusions by LM
MERS = Middle Eastern Respiratory Syndrome Coronavirus (MERS-CoV)
- appeared in Summer 2012 in Arabian peninsula
NL63 and HKU1: new agents of LRTI
12. Arenaviridae
L. "arena‟ = sand; sandy appearance of virion; from
rodents, tx aerosolised urine, feces (like Hanta)
Lymphocytic choriomeningitis virus
Hemorrhagic fever viruses: Filo, Arena, Flavi, Bunya families
- Lymphocytic choriomeningitis virus,
- Lassa viruses; Viruses of the Tacaribe complex
“New World Arenaviruses”
Ebola Virus
Ebola Hemorrhagic Fever
• Severe, often fatal disease in humans and nonhuman primates (monkeys, gorillas, chimpanzees)
• Initial recognition 1976, due to Ebola virus, named
from Ebola River in Zaire
• ssRNA enveloped virus, family Filoviridae,
unknown animal serves as natural reservoir in
Africa (monkey?)
• Confirmed cases in Africa, one lab worker England
via needle-stick, no cases in USA; monkeys ill and
died in VA with Ebola-Reston subtype, had been
imported
• Transmission via blood and body fluids;
amplification within health-care setting during
outbreaks
• Abrupt onset: fever, H/A, joint and muscle pain,
weakness, diarrhea, vomiting, stomach pain, rash,
red eyes, followed by the signs of hemorrhagic
fever viruses: internal and external bleeding due
to damage to vascular endothelium
• Laboratory diagnosis: serology EIA IgM and
IgG, RT-PCR, virus isolation in BSL-4 facility
• No standard treatment


Measels (Rubeola)

Rabies virus

13. Retroviridae
L. "retro‟ = to go back; Have an RT (reverse transcriptase): RNA-cDNARNA
- Retroviruses contain the reverse transcriptase enzyme,enveloped ssRNA viruses with icosahedral symmetry and contains spiked knobs
● Transmembrane protein, TM or fusion protein, also called gp41
● Surface protein, SU or attachment protein, also called gp120
● gp120 binds to a cell receptor to initiate infection
● Icosahedral core is cone-shaped
● Core contains the major capsid protein, CA or p24
● Reverse transcriptase, Integrase, and Protease are also found within the capsid
Human T-cell Lymphotrophic Virus (HTLV) 1 and 2
● 1st human retrovirus discovered, 4 HTLVs known
● HIV was first classified as a HTLV
● Both HTLV-1 and HTLV-2 affect humans, CD4+
● Six different subclasses of HTLV-1
● Each subtype endemic to geographic region
- seen in s Japan, Caribbean, S Africa, Brazil
● HTLV-1 infection primarily in immigrants, children of immigrants, sex workers, and IVDU
● Prevalence increases with advancing age
Transmission
● Breastfeeding & childbirth, Sexual contact, Transfusion, Transplant, IVDU (linked to HTLV-2)
Sx
● Most infections are latent or asymptomatic
● HTLV-1 is associated with two diseases
Adult T-cell leukemia (ATL)
● Symptoms are clinically broad but include; fatigue, overt lymphadenopathy, thirst due to hypercalcemia, nausea, vomiting, fever, abdominal pain, skin rash, intense thirst, and inc free IL-2 receptor in serum
● develops in 2-4% of HTLV-1 infected individuals
● Acute form comprises 55-75% of all ATL cases
● significantly increased white cell count
● generalized lymphadenopathy
● Chronic form → absolute lymphocytosis that persists for months to years
● Smoldering ATL → 5% or more abnormal T lymphocytes with a normal total lymphocyte count
Myelopathy/tropical spastic paraparesis (HAM/TSP)
● Develops in 1-2% of HTLV-1 positive patients
● Slowly progressive degenerative disease affecting the corticospinal tracts of the thoracic cord
● degeneration and fibrosis in the spinal cord
● Also-->HTLV-1 is associated with uveitis (eye inflammation) and HTLV-1 associated infective dermatitis.
HTLV-3 (HIV-1 and -2)
a. Initial infection → HIV infects CD4+ Tcells
b. Acute phase viremia → HIV replication in CD4+ cells and lymph node involvement
c. Latent period → The number of CD4+ cells slowly declines
d. Clinical complications develop during latent period
e. Progression to AIDS → CD4+ cells rapidly decline accompanied by a loss of immune capacity
f. End-stage AIDS → All systems of the body are affected by the HIV itself and a variety of opportunistic infections
Dx
ELISA screens for HIV, sens >99%, usually false neg in first few mo of infx (still doesn't have anti-HIV Ab's and insensitivity to HIV-2 and HIV-1 subtype O)
- confirmation c Western blot (~100% spec; gel electrophoresis c known HIV proteins blotted on nitrocellulose paper and pt serum added, which can form bands); false-neg c subtype O and false-+ c hyperbilirubinemia
- p24 "capture" detectable early
- CD4 count by flow monitors progression; q 6 mo
- proviral DNA (2/2 reverse transcriptase on cDNA) can be used for early dx, relatively poor sens (95%) and spec (98%)
- viral load of HIV RNA important in management
-- predicts long-term outcomes, dz progression, and medication needs
-- can be measured by RT-PCR, branched DNA (bDNA), nucleic acid sequence-based amplification (NASBA); should only use 1 method to follow pt
- HIV should be dx'd early, as HAART initiation greatly beneficial early, best to use HIV RNA

References
1. CLS lecture notes
2. Osler notes.
