Uterus
Embryology, Anatomy and Histology
Arias-Stella reaction
Atrophy
Placental site nodule
Placental polyp
Placenta accreta
Dysfunctional Uterine Bleeding (DUB)
Endometritis
Endometrial polyps
Endometrial Hyperplasia
Endometrial carcinoma
-
Endometrioid carcinoma
- Adenosquamous carcinoma
Clear cell carcinoma
Endometrial Intraepithelial Carcinoma (EIC)
High-Grade Neuroendocrine Carcinoma (HG-NEC)
Mucinous Adenocarcinoma
Serous carcinoma
Lymphoepithelioma-like carcinoma
Transitional cell carcinoma
Carcinoma with trophoblastic differentiation
Undifferentiated Carcinoma
Mixed Tumors
- Adenofibroma, adenomyoma, Atypical polypoid adenomyoma, Adenosarcoma, Malignant mixed mullerian tumor (MMMT, carcinosarcoma)
Pure Mesenchymal Neoplasia
- Neoplasm arising from endometrial stroma, Lesions arising from smooth muscle (usual type), Epithelioid / Myoid smooth muscle neoplasms, Uterine tumors resembling ovarian sex cord tumors (UTROSCT), rhabdomyosarcoma, hemangioma, angiosarcoma, lipoma, liposarcoma, adenomatoid tumor, Perivascular epithelioid cell neoplasm
Lymphoma
Carcinoid
Primitive Neuroectodermal Tumors
Uterus
Embryology
Paramesonephric ducts form main female genital ducts, that start as a vertical cranial part that opens into abdominal cavity, a horizontal part crossing the mesonephric duct and a caudal vertical part that fuses c contralateral side; the first two form the uterine tube and the caudal part the uterine canal
- ureterorectal pouch and uterovesical formed by uterus and broad ligament; and the fused paramesonephric ducts form corpus and cervix of uterus
- ovarian gubernaculum relocates ovaries from their position on posterior abd wall, and forms the ligament of the ovary and the round ligament of the uterus

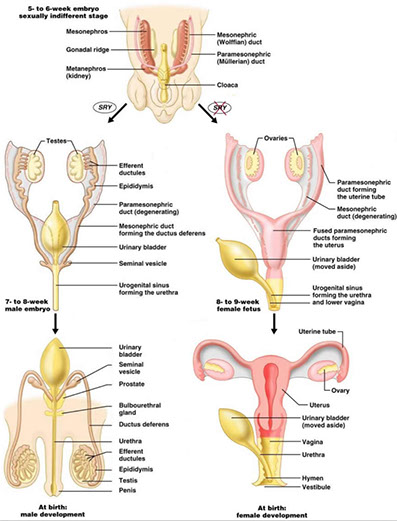
Anatomy
size and shape vary c age
- anteverted is tipping forward, andtiflexed is bent forward
- body of uterus is superior 2/3 and includes the fundus, ends at the isthmus of the uterus at the cervix, the inf 1/3
- body of uterus divided into perimetrium (serosa or outer serous coat of CT), myometrium (middle coat of sm muscle), and the endometrium (inner mucous coat shed in menses)
- pelvic diaphragm helps support the uterus
- peritoneum covers the uterus anterior and superior
-- vesicouterine pouch separates uterus from bladder anteriorly by a peritoneal reflexion
- similarly the uterus is separated from rectum by a peritoneal rectouterine pouch
- blood supply from uterine arteries from internal iliacs, c collateral from ovarian arteries
Histology
Endometrium has simple columnar epithelial lining c assoc simple tubular endometrial glands and endometrial stroma (the lamina propria)
- made of 2 layers: functional layer (affected by hormones and blood supply) and a basal layer (not affected, has pretty constant look of weakly prolif glands and cellular spindled stroma)
- Lower Uterine Segment (LUS) is thinner and less hormone-responsive than the body; glands may appear more endocervical and the stroma is more fibrous than the uterus and more cellular than the cervix
- EMB not done to assess fertility anymore
- glandular crowing can artificially occur as tangential cut of basalis layer and look hyperplastic, called "stack of coins"
- hobnail artifact, look like non-pleomorphic cells (vs serous ca), tufting, possible mits,
- similarly, heat can artifactually cause tufting
- can see telescoping in glands
- endocervical tissue can be seen in endometrial bx, must distinguish b9 (EC microglandular hp) from cancer (EM AC c microglandular-oid look)
- may see follicular cells in bx, possibly luteinized c IHC of sex cord-stromal cells (+ calret, INH, CD56, WT1, hormone r's)
- pseudolipomatosis is group of variably-sized spaces that and stroma clear of capillaries or interstitium, confirm c S100 neg (vs adipocytes that stain unevenly for S100)
- finding fat in a bx indicative of uterine perforation, usually 2/2 currettage procedure (call the clinician! Pipelle bx usually asx even if perforated)
Lower Uterine Seg

Endometrial Stroma
- May see atypical (bizarre) cells, esp in endometrial polyps and sometimes in prolif endometrium; c inc NC and dark nuclei, no inc mits, occasional multinucleation; (+) ER/PR, androgens, CD10, SMA
- lymph aggs sometimes in basal portion
- foamy cells can be macrophages or cells s/p E stim, c fine vacuoles, small round folded central dark nuclei, can form nodular histiocyte aggs, may be signet ring, irreg nuclei, inc mits, (+) CD68
Menstrual cycle divided into proliferative and secretory phases
- 28 days by the book (day 1 is menses), but proliferative phase is varied, while the secretory phase is constant
Atypical cells in endometrial stroma

Proliferative phase
Much more variable than secretory phase, regeneration of endometrium that has sloughed off from last menses
- endometrial glands lined by pseudostratified columnar c dense chromatin and small nucleoli
- early in this phase (d4-7) the surface endometrium is thin and orderly
- in mid-phase (d8-10) the glands are a little more zigzag and surface is taller columnar, mits in stroma and epithelium
- late-phase - glands c wavy surface and more coiled glands, more abundant mits in epithelium and stroma, no more stromal edema
Interval Endometrium
Up to 36 hrs after ovulation has scattered glands c subnuclear vacuoles c pseudostratification and mits
proliferative endometrium

Secretory phase
Dating based more on stromal changes
- early phase - Post-Ovulatory Days (PODs) 2-5 (16-19 of entire menstrual perior, see vacuolated endometrial glands, subnuclear vacuoles in >1/2 indicate ovulation occurred up to POD4 where they become supranuclear as nuclei become more basal, and glands are more pseudostratified
- mid-secretory phase - POD 9-14, spiral arteries more prominent from surrounding stromal condensation; POD10 glands have more sawtooth edges and stromal edema diminishes; POD 12 PMNs appear and sheets are formed from prominent predecidual changes; fibrin thrombi and stromal hemorrhage on POD 14
b9 early secretory endometrium, looking like piano keys

Menses
Glandular and stromal breakdown c glandular crowding
- ovulation shows uniform glands c subnuclear vacuoles
- in exodus the shed endometrium forms a ball with the endometrial epithelium on the outside and stroma on the inside
Gestational endometrium
On implantation, see few changes, like glands c distention from intraluminal secretion and cytoplasmic vacuolization, decidualized stroma around vessels, a little Arias-Stella
- 2 WGA stroma more decidualized
- >4 WGA glands atrophy 2/2 inc decidualization, some necrosis in stroma (at end of pregnancy, glands are very atrophic and dilated, hard to distinguish from BVs)
- chorionic villi after 12-13 WGA, at first c few BVs and lots of stromal edema, lined by outer syncytiotrophoblast and inner cytotrophoblasts
- chorionic villi more plentiful after 13 WGA
- implantation site of the placenta has fibrous tissue and intermediate trophoblasts infiltrating decidualized stroma, spiral arteries and even into the myometrium, hPL+
- need to see villi, trophoblasts or fetal parts to dx intrauterine preg

Exodus of the endometrium with endometrial epithelium on outside and stroma on inside
Arias-Stella reaction
Hormonal changes assoc c pregnancy (inc progesterone), usually after 2 WGA
- usually a focal, alternating atypical cells c normal appearing cells
- endometrial glands c lots of vacuolated red cytoplasm c large, dark or possibly cleared-out (2/2 biotin accumulation) nuclei c pleomorphism and invaginations, hobnailing, possible rare mits
- no nuclear molding or necrosis (vs viruses)
IHC:
- negative Ki67 (low), p53

Arias-Stella rxn
Atrophy
Normal in premenarchal and postmenopausal periods
- assoc c bleeding (postmenopausal)
- micro: low columnar to cuboidal or flat epithelium c inc NC, or ciliated / tubal metaplasia, possible crushed stroma
endometrium c atrophic glands in decidualized stroma from exogenous progesterone

Atrophy; endometrial gland without mitotic activity

Placental Site Nodule
Well-circumscribed lesion of intermediate trophoblastic cells in hyalinized matrix, can have dark scary, pleomorphic cells
- mits infrequent, binucleate cells common
IHC: similar to SCC, (+) CK, EMA, and p63, but is CD10, inhibin and HLA-G +, sometimes Mel-Cam+
- neg: p16
Placental Polyp
Retained chorionic villi from incomplete abortion that looks like a pedunculated polyp to the clinician
- micro: necrotic, hyalinizaed or focally calcified, may see trophoblastic cells


Glandular and stromal breakdown, stromal condensation and fibrin deposition
Glandular and stromal breakdown, papillary syncytial metaplasia
Placenta Accreta
Dx'd by seeing villi next to myometrium w/o intervening decidua but c hyaline stuff and scattered intermed trophoblasts
Dysfunctional Uterine Bleeding (DUB)
2/2 changes in normal endometrial cycles
- does not include postmenopausal bleeding or bleeding 2/2 any known condition
- micro: usually stromal and glandular breakdown
- stromal collapse c stromal cells condensing, fibrin clots, glandular disruption, papillary syncytial metaplasia
- glands prolif to weak secretory
- epithelial hyalinization or reactive atypia
- unusually, stomal cells may appear signet ring
Estrogen-assoc bleeding
- in anovulatory cycles see prolif pattern c glandular/stromal brekdown; MC in pre-/perimenopausal pts, occasionly in reprdctve yrs
- grade of prolif chnges related to time of stim c unopposed E (more proliferative c subnuclear vacuoles in chronic anovulation, less so in acute)
Progesterone-assoc bleeding
- glandular / stromal breakdown c abnormal secretory pattern in perimenopausal and reproductive age women
- may be 2/2 irreg shedding and luteal phase defects, but may be unknown on histo exam
Luteal phase defect
- controversial, secretory endometrum failure to mature 2/2 corpus luteum problems
- assoc c recurrent abortion and infertility (not proven c data)
Irregular shedding - uncommon cause of DUB; assoc c persistent corpus luteum and prolonged P production
- micro: mix of secretory / prolif glands in sample taken at least 5 days after start of bleeding
Underdeveloped secretory endometrium c glandular and stromal breakdown

Hormonal Effects
Atypia in hormonal tx
can see big dark nuclei, red cytoplasm, in em c tamoxifen, combined HT, norethisterone
- possibly scattered p53+ or focal inc in Ki67, but usually neg c p16
Estrogen
- inc risk endometrial AC c unopposed exogenous E, usually in perimenopausal women trying to control sx (hot flashes, vaginal atrophy, bone breakdown)
- ex.: Premarin, ethinyl estradiol, diethystilbestrol
- micro: prolif endometrium (em), disordered prolif em, atypical hyperplasia, AC
- risk high after 2-3 yrs E, highest at 10 yrs
Estrogen effect on endometrium showing glandular hypertrophy

Progesterone and OCPs
-used in P-only therapy to tx DUB, or in low-grade em-oid AC c IUD or hypodermic implants
- ex.: medroxyprogesterone acetate or norethindrone acetate (supress ovulation and em growth and induce secretory maturation and P withdrawl bleeding)
-- can be given in small constant doses or incremental, but usually have 21 days hormone c 7 days break in hormone
- P-oid em in perimenopausal women can be seen w/o having taken pills, possibly 2/2 "luteinized unruptured follicle" or persistent corpus luteum
- 3 micro patterns:
1) decidual "preg-oid" - usually after high P tx given for anovulation or em hyperplasia, abundant polypoid tissue, stromal decidualization c some mits, glands hypersecretory c cytoplasmic vacuoles c luminal secretions or atrophic
- possibly Arias-Stella or sq metaplasia, superficial ectatic venules
2) underdeveloped secretory - looks atrophic slightly coiled to straight, inactive irreg glands in lots of (possibly edematous or decidualized) stroma, intraluminal secretions
3) Atrophic - straight glands lined by inactive epithel c basal nuclei and scant cytoplasm, possibly weak secretory, cellular stroma c big cells or edema, ectatic BVs
Combined E+P for HRT
- em c weakly prolif, sometimes combined c secretory, atrphy or focal brekdwn can be seen
Tamoxifen - despite antiestrogenic on breast, has mild E effect on em, assoc c em hyperplasia, polyps, metaplasia and CAs/sarcoma
Raloxifene - 2nd gen selective E r modulatoranti-E in breast ca, and for osteoporosis prevention, weak E agonist on em not assoc c pathologies, usually atrophic em
Clomiohene citrate - anti-E that induces ovulation in infertile or those c ovulation disorders
- em bx done in luteal phase to assess tx response
- should see secreotry pattern, can have stromal decidualiaion, dec glands (size and #) or secretory activity or out of phase
- may induce luteal phase defect, except in pts c PCOS (?)
Danazol - steroid c weak P, used to tx endometriois, menorrhagia, and em hyperplasia
- em usually atrophic, but can be weakly secretory
Human Menopausal Gonadotropins (hMGs) / menotrophins - seen in peepee of postemnopausal; contain FSH and LH, used to induce ovulation in anovulatory pts
- hCG used c hMG or clomiphene citrate to stim and better midcycle surge of LH assoc c ovulation
- may cause glandular / stromal dyssynchrony, or be normal
Gonadotropin-releasing Hormone Agonists - leuprolide acetate, busesrelin acetate, goserelin acetate
- used to dec size of leiomyomas b4 srgery, to suppress em b4 resectoscopic blation, prevent spontaneous LH surge b4 oocyte retrieval, improve development of follicle for IVF and gamete intrafallopian transfer
- micro - suppresses em, or is weakly prolif, atrophic
-- may have P effect if given c OCP`
Progesterone effect on endometrium showing decidualized stroma

Endometritis
Acute or chronic
- acute usually seen after preg (term or abortion), instrumentation or Group A strep c inc neuts in glands and surface em c microabscesses
- chronic in perimenopausal / older women, may be 2/2 bacteria (strep, Enterococcus, E coli, mycoplasma), retained placental fragments, PID, IUDs, instrumentatio, cervical stenosis, polyps, leiomyomas, em hyperplasia, ca
- may see reative atypia, glands in any phase, necrosis, eos, spindly stroma, intraluminal inflam, focal breakdown, plasma cells (may stain?)
Chlamydia trachomatis - mod to severe c lymph, plasma cells, neuts, necrotic foci, use urine / vaginal / cervical swabs to detect DNA / RNA
Actinomyces israelii - assoc c IUD (1/5), mostly asx, but can have variety of inflam, sulfur granuls (bacterial colonies [GMS+] c tissue and Ca phosphate)
- must differentiate from pseudoactinomycotic radiate granules in pts w/wo IUDs, which are refractile, red, c peripheral clubs in radiate shape and blue Ca lines that look like tidewater marks (GMS neg)
- Splendore-Hoeppli phenomenon assoc c Actinomyces shows red eosinophilic globules around actinomyces, site of ag-ab interaction
Cytomegalovirus (CMV)
Uncommon, assoc preg / IC (transplant pts/AIDS)
- micro - big blue intranuclear inclusion en em epithelium, acute (eos) and chronic inflam
Herpes - em-itis rare 2/2 herpes, seen in IUD, IC, after preg
- assoc c acute inflam and focal necrosis, ground glass nuclei c molding, large Cowdry A rec nuclear inclusions
- trophoblasts can have clearing in nuclei and Arias-Stella may look similar, may do herpes IHC
Mycoplasma - patchy, subtly lymph aggs, macros, few neuts, rare plasma cells, best seen mid-secretory, possibly granulomatous rxn
Granulomatous inflam may be 2/2 rads, foreign body, tb (noncaseating granulomas), sarcoid, em ablation tx, or other infx
- can have necrotizing granulomas surriounded by lymph / eos after em ablation, possibly c hemosiderin
- fungi can also cause granulomas!
Eosinophilic Endometritis - may persist up to 21 days after em curettage
Pneumopolycystic Endometritis - very rare, self-limiting, can affect anywhere from tubes to vulva, has well-defined empty spaces in em stroma surrounded by thin compressed stroma c rare macros
- may look similar to gas gangrene (Clostridium), preg, processing, cancers
Chronic endometritis

Actinomyces assoc c IUD infx

Splendore-Hoeppli phenomenon assoc c Actinomyces shows red eosinophilic globules around actinomyces

Endometrial Polyps
usually in 40-50s yo, assoc c bleeding, infertility, tamoxifen, usually single but may be multiple
- esp in postmenopausal, can see ca, sarcoma, em hyperplasia
- micro - thick BVs, glands irreg spaced / shaped, out of phase (underdeveloped glands), glands arranged parallel to long axis of surface epithelium
- stroma may be scarce, giving the polyp a hyperplastic appearance, look for thick BVs
- atrophic in postmenopausal, c dilated glands c low cuboidal epithelium, fibrotic stroma
- can be functional if they have prolif / sec changes in response to hormones, should compare c background epithelium
- mixed polyps c endometrioid- / endocervical-like epithel in fibrous stroma, may arise in LUS or upper EC canal, but can be from anywhere and can see mucinous metaplasia in postmenopausal
- myomatous if lots of muscle
- other metaplasias (sq, ciliated, mucinous, eosinophilic, hyperplastic) possible and ablation can cause worrisome features
- polyps larger + multiple c tamoxifen tx, have staghorn glands, ca
- atypical (bizarre) cells seen, but have low mits
Radiation Effects
Granulomatous inflam, em epithelial atypia c large pleomorphic nuclear changes, cytoplasmic vacuoles, xanthogranulomatous em=itis similar to cervical canal blockage c bacterial infx
- also see lots of foamy histiocytes, some MNGCs, chronic inflam, necrosis, Ca and hemosiderin deposits, cholesterol clefting
Endometrial Hyperplasia
Generally, glands > stroma (>3:1 G:S ratio by area; [2:1 seen in prolif em])
- risk factors: obesity, nulliparity, unopposed estrogen, polycystic ovaries, DM, HTN
- Classified by WHO: Hyperplasia without atypia (previously simple endometrial hyperplasia), or Atypical hyperplasia / endometrioid intraepithelial neoplasia (or complex endometrial hyperplasia)
- micro: some glandular pseudostratification and inc mits seen
Simple Hyperplasia
Prolif of variably-sized em glands without outpouchings
Complex Hyperplasia
Markedly irreg glands c freq outpouchings
Atypia
Large round (not oval) nuclei, irreg chromatin distribution, variable nucleoli - should be present in a large % of cells and easily found, not 2/2 artifact
- metaplasia doesnt mean atypia
- secretory hyperplasia seen in perimenos tx'd c P, EP, or GnRH, or idiopathic, c crowded disorganized glands c variable cytoplasmic vacuoles and luminal secretions, possible decidualized stroma, must compare to background em
Endometrial Intraepithelial Neoplasia (EIN)
- assoc c large inc risk em ca, monoclonal preinvasive glandular prolif, c architectural alterations (glands crowded [>50%], but overall pattern not important), cytologic alterations (classic atypia not necessary),must be >1 mm, must not be a mimicer (polyps, repair, metaplasia), not ca
Metaplasia
- differentiation changes or regular / prolif secretory em epithelium
- squamous metaplasia - ranges from highly keratinized to morular c immature spindly form c indistinct cell borders c amphophilic cytoplasm without keratiniztion; may be assoc c chronic irritation
- ciliated (tubal) metaplasia - ciliated cells normal in em surface epithelium esp in prolif phase; but can be seen in em glands c unopposed E; can have round nuclei (though is not atypia or hyperplasia); IHC shows wild-type p53 and low Ki67
- eosinophilic metaplasia - columnar, cuboidal or slightly round cells c lots of red cytoplasm and somewhat round nuclei c even chromatin, may have cytoplasmic mucin; assoc c atrophy, prolif, em hyperplasia and em-oid AC
- papillary syncytial metaplasia - assoc c breakdown and bleeding assoc c degeneration and regeneration, looks like bland red cell in small papillae w/o fibrovascular core; if cells too atypical can dx serous ca; interpret IHC c caution
- mucinous metaplasia - abundant mucin in cytoplasm in em cells, may be a precursor to mucinous AC of em (4/5 +KRAS)
- hobnail metaplasia - may be assoc c currettage, abnormal bleeding or radio-tx
- clear cell metaplasia - uncommon, has clear cytoplasm w/o mucin or glycogen by IHC
Hyperplasia may be mimicked by endometrial atrophy c cystic change, polyps, endometritis, disordered prolif em, em AC FIGO 1, serous ca, artifact on secretory em, papillary prolif of em (possible assoc c ca), other artifacts (fragmentation, improper orientation, telescoping, hobnailing), contamination from endocervix
Px / Tx - regression in 4/5 c em hyperplasia w/o atypia, persists in 1/5
- 1/100 c simple and 3/100 c complex em hyperplasia get ca
- 1/10 simple c atypia and 3/10 complex c atypia get ca
- em hyperplasia and even em-oid AC FIGO1/2 can be tx'd c exogenous P or GnRH and have em sampled q 3-6 mo to follow progression (expect to see decidualized glands c dec G:S ratio and eosinophilic metaplasia)
-- tx has failed if atypia persists 6 mo s/p tx; may also worsen dz
Endometrial hyperplasia, nuclear pseudostratification. Note the mit (arrow)


Disordered Proliferative Endometrium
Common and normal pattern in perimenarchal and postmenopausal years
- functionally correlates with anovulatory cycles and exogenous estrogen
- no inc risk of endometrial carcinoma
Resembles normal prolif tissue c glands lined by cytologically bland, pseudostratified proliferative, mitotically active epithelium and c roughly normal ratio of glands to stroma
- normal prolif endometrium from synchronous and coordinated growth of fundal functionalis under influence of estradiol
- disordered prolif endometrium, however, is from dissynchronous growth of functionalis
- in some areas glands can be cystically dilated or have shallow budding, and in some areas glands are tubular of narrow caliber and in abundant stroma
- metaplastic epithelium (esp ciliated) is often encountered
- no significant cytologic atypia is present
- evidence of recurrent endometrial breakdown and hemorrhage can be present c thrombosed, thin-walled vessels and interstitial fibrin and hemorrhage
- should have normal ratio of glands to stroma (~1:1) whereas hyperplasia has glands to stroma ratio of 3:1
-- disordered proliferative endometrium can be thought of as the bridge bwt normal prolif and hyperplasia
Disordered proliferative endometrium

Diagnostic algorithm for endometrial carcinomas. Abbreviations: AMACR, a-methylacyl CoA racemase; ER, estrogen receptor; FIGO, International Federation of Gynecology and Obstetrics; H&E, hematoxylin-eosin; HER2, human epidermal growth factor receptor 2; HNF-1b, hepatocyte nuclear factor 1b; MMR, mismatch repair protein; PR, progesterone receptor; SW1/SNF, switch/sucrose nonfermentable. [3]
Endometrial carcinoma
MC gynecologic malig in USA (33k cases, 4k deaths)
- postmenopausal c sx of bleeding
- assoc c prolonged E (w/o P), DM, HTN, infertility, Muir-Torre syndorme, Stein-Leventhal syndrome, Turner's syndrome
- spreads / mets to cervix (directly or seeded by D+C), ovary, vagina, bone, CNS, liver, lung, skin
-- mets to LN (pelvic and para-aortic)
- 1/10 assoc c ovarian ca, which has same histo features
- must differentiate from cervical or other site of origin
- IHC: (+) vimentin, ER, patchy p16 (can be diffuse in high-grade), neg CEA
- EC AC p16 diffuse +, CEA+, neg vimentin, ER
- LUS can have mixed IHC
- type 1 is the endometrioid type, and type 2 is the higher-grade types
Differentiating endometrial from endocervical carcinoma
Difficult to do by morphology alone
- usually what you see is low- or intermediate-grade glandular prolif of stratified columnar mucin-poor cells, which raises the differential of usual type endocervical vs endometrioid endometrial AC
- endometrioid AC arising in the endocervix is not that common (5% of all endocervical AC)
- endocervical AC of usual- and endometrioid-type almost always assoc c HPV, and thus usually show diffuse p16 staining due to inactivation of Rb by HPV E7 protein
- endometrial endometrioid AC is negative or only focally and weakly p16+ in the glandular component, though areas of squamous differentiation can have more diffuse staining
- CEA, vimentin, ER/PR may also be helpful
- a significant number of tumors may not be differentiated with the help of IHC, in which case high-risk HPV ISH or PCR may help
Lynch syndrome assoc c inc risk, esp c MSH6 carriers (up to 3/4)
- tumors usually more heterogenous, and more often from LUS
- 1/2 of cases have endometrial carcinoma as the presenting ("sentinel") cancer event
- consider esp if clear cell type in younger patients
- though is only about 1/20 endometrial cancer, will need inc screening for cancers
- microscopic features: poorly diff, mucinous features, signet ring cells, mixed tumor histology, tumor infiltrating lymphs, Crogn-like inflam infiltrate invading at periphery
Several subtypes per WHO:
Differentiating bwt different subtypes of endometrial adenocarcinoma (EAC) has major clinical and prognostic implications, so if a precise pathologic dx cannot be made on morphology alone, IHC should be used to classify
Subtypes (see below for more info on some subtypes):
Endometrioid ca (secretory, villoglandular and secretory variants)
- usually have a wild-type p53 immunostaining pattern, which is weak focal staining in tumor cell nuclei
- a small portion of FIGO grade 3 endometrioid EACs have shown p53 overexpression and is usually assoc c adverse clinical outcomes
- p16 weak and patchy
Mucinous ca
Serous ca
- up to 90% of cases has p53 mutations, which correlates with abnormal p53 expression by IHC
- usually p53 has diffuse and strong nculear staining in >75% of tumor cells (only 10% of serous EACs show complete absence of p53 immunoreactivity due to nonsense mutations or homozygous gene deletion
- p53 mutation is an early event in serous carcinogenesis
Clear cell ca
- usually have a wild-type p53 immunostaining pattern, which is weak focal staining in tumor cell nuclei
- p16 negative
High-grade neuroendocrine ca (small and large cell types)
Undifferentiated ca
Mixed ca
- others
Micro: needs to invade myometrium to dx (but be careful bc can be overdiagnosed if endomyometrial junction is atypical or with adenomyosis (back to back glands should be at least 2 x 2 mm)
- stroma should have desmoplasia or fibroblastic rxn in area infiltrated by glands disappearing (or necrotic; more cribriform glands)
- lots of atypia in high-grade
- stromal foam cells bwt glands suggestive but not diagnostic of ca
- stratified columnar cells c luminal mucin
Tx: TAHBSO, possible nodal dissection
- P causes regression if well-diff, but doesn't cure
- chemo if high-risk early stage, or late stage and recurrences
Px: FIGO stage, histo type (clear cell and serous are bad), grade, angiolymphatic invasion (esp if stage 1), angiogenesis EGFR (assoc c microscopic grade and shortened survival time), ER, p53 overexpression, HER2, DNA aneuploidy (seen in 1/4 cases, usually at a later stage)
-FIGO staging discussed below



Villoglandular adenocarcinoma of endometrium, will have wild-type p53 (vs diffuse positivity in serous ca)

Clear cell carcinoma of the endometrium

Endometrioid (endometrial) carcinoma
aka type I endometrial carcinoma
MCC endometrial ca (4/5), Indolent cancer due to endometrial hyperplasia
- seen post-menopause white women, after complex atypical hyperplasia, assoc c hormone replacement (E without the P)
Micro: em glands back-to-back >0.2 x 0.2 cm, papillary pattern extensive, or infiltrating irregular glands c desmoplastic or fibroblastic stromal rxn (though this may be reactive... be careful)
- rounded glands have mild-mod atypica c pseudostratification
- can see mucinous differentiation, necrosis in the glands, ciliated cells, and foamy histiocytes in the stroma
Squamous differentiation in Endometrioid AC
Micro: rounded intraluminal masses (morula formation, +CDX2), SCC-oid nests, squames c clear cytoplasm from glycogen, papillae assoc c mucinous metaplasia
Villoglandular differentiation
Micro: papillae lined by columnar cells c bland oval nuclei
- may be assoc c inc rate of lymphovascular invasion and LN mets
Secretory differentiation
Micro: has intracytoplasic glycogen
IHC: inc Ki67, but has good px
Ciliated cell changes
Although glands ciliated, acts like an em-oid ac
Clear cell changes - usually only focal, possibly 2/2 fat
Sarcomatoid (spindle cell) features - keratin staining and border c regular em-oid ac help to distinguish from MMMT
Microglandular pattern - assoc c hormone use
- some of the glands can be large and cystic, most are small and have some solid foci, has lots of luminal secretions c neuts
Small nonvillous papillae - low nuclear grade, various gland complexity, does not change px
Sertoliform pattern - tubules c clear columnar cells, EMA+, rarely inhibin +, no change in px
Sex cord-like formation and hyalization - may be mistaken for MMMT, has cords / clusters of epithelioid to spindled cells mixed c em-oid AC; hyalinized matrix can look like osteoid or small spherules, EMA usually <50% of cells, Ki67 too low to dx MMMT
Oncocytic change - may see cells c lots of red cytoplasm (from mitochondria), can be low or high grade
IHC: (+) PAX8, ER/PR (9/10), CA125, low Ki67, vimentin
- neg: p16 (patchy), p53 (patchy, rare weakly pos cells), HNF-1beta, WT1, CEA
Genes: microsatellite instability (in 1/4), PTEN (>1/2), PIK3CA (1/3), PIK3R1 (>1/3), ARID1A (4/10), TP53 (1/3, in higher grades), k-ras
- DNA aneuploidy seen in 1/4 of cases and assoc c advanced stage
Tx: Hyster, add rads if myoinvasive
- bx before surgery determines initial surgery plan, but this can change c IOC performed during surgery
Px: staged on depth of invasion, age, but can also use FIGO grade (based on % that is solid)
Grading
- FIGO 1 (well-differentiated) - complex glands with little intervening stroma; <5% solid, nonmorular sheets
- FIGO 2 (moderately differentiated) - 5-50% of tumor is sheet-like without glandular appearance
- FIGO 3 (poorly differentiated) - >50% tumor made of sheets of cells
-- squamous morules not counted in solid areas (??)
Stage 1 = 90% survival @ 5 yr, Stage 3/4= 20%
- with serous, clear cell and SCC, nuclear grading is more important; also if there is lots of nuclear atypia raises grade by 1
Px also dependent on microscopic type (clear cell and serous ca c poor px), microsatellite instability, p53 overexpression, lymph-vascular invasion, angiogenesis, and inc EGFR expression (assoc c microscopic grade and shortened survival time)
Ki-67, survivin expression, p21 assoc c px
- if invades >50% of depth of uterine wall on rapid frozen section (RFS), surgeon will do inguinal LN staging
Endometrioid endometrial adenocarcinoma (A) can be differentiated from an endocervical primary by weak and patchy p16 expression (B), strong diffuse vimentin expression (C), estrogen receptor (ER) positivity (D), and carcinoembryonic antigen (CEA) negativity (not shown) (hematoxylin-eosin, original mag x4 [A]; original magnification x4 [B through D]).



Endometrioid carcinoma, FIGO grade 1

Histology and immunohistochemistry of endometrial endometrioid carcinoma. Endometrial endometrioid carcinoma shows a glandular growth pattern (A and B), a wild-type p53 expression (C), patchy p16 staining (D), diffuse nuclear staining for estrogen receptor (E) and progesterone receptor (F), focal nuclear positivity for HNF-1b (G), and negativity for napsin A (H) [3].
Adenosquamous carcinoma
An endometrioid adenocarcinoma c at least 10% squamous parts; possibly 2/2 metaplasia
Micro: Definite keratinized squamous component c intercellular bridges and / or 1) gland / palisading in sheets, 2) clear-cut margins, 3) red, thick or glassy cytoplasm, 4) dec NC compared to rest of tumor
IHC: (+) CD44 in squamous part
Px: Same as adenoca
Squamous Cell Carcinoma
Rare (<1/100); to ddx must r/o em ca co-existence or cervical scc
- assoc c cervical stenosis, uterine prolapse, chronic pyometra, rads hx
- usually bland
- px: good if confined to uterus, bad if stage III
- IHC: neg ER/PR
Clear cell carcinoma
Occurs in older women, 1/20 em ca's
- no relation to hormone exposure
- high grade by definition
Micro: red or clear glycogen-filled cuboidal cytoplasm with distinct cell borders in papillary, tubulocystic or solid patterns
- variably atypical nuclei, but usually pretty weird, variable mits
IHC: (+) HNF-1beta, Napsin A, p504s, intermediate to high Ki67,
- neg: WT1, p53 (var), ER/PR (var, usually PR neg)
- p16 negative (usually)
Px: poor

Clear cell (A and B) and endometrioid (C and D) endometrial adenocarcinomas have wild-type (ie, focal, weak) p53 expression patterns (hematoxylin-eosin, original magnification x10 [A and C]; p53, original magnification x10 [B and D]).
Endometrial Intraepithelial Carcinoma (EIC)
May be a precursor lesion to serous carcinoma
- may met
- assoc c p53 mutation
High-grade Neuroendocrine Carcinoma (HG-NEC)
Rare (more common in cervix); Small or large cell type; various architectures (occasionally has rosette-like structures), usually assoc c em-oid AC
- small cell type has round or spindly cells c min-mod cytoplasm, uniform nuclei w/wo nucleoli
- large cell has lots of cytoplasm c variable-sized vesicular or hyperchromatic nuclei, occasional nucleoli, prominent mits, geographic necrosis, neuro-vascular invasion
- IHC: + CK, EMA, CAM5.2, CD56, SYN, S100, p16
- px aggressive (<1 yr survival)
Mucinous Adenocarcinoma
em ac c >1/2 mucinous cells
- various patterns *glandular, villoglandular, solid, villous
- mild or mod atypia (rarely marked), no prominent mits (0-9/hpfs)
Serous carcinoma
1/10 em ca, mod to marked atypia in papilary, glandular or solid patterns
- assoc c atrophic endometrium and not with hormone exposure seen in older black women; may be assoc c some tx's (chemo-rads) and other gyn ca's
Micro: looks and acts like serous ca of the ovaries (aka papillary serous ca), with papillae having lining cells c lots of atypia with multinucleation, "lobster-claw" branching, detachment of cancer cells into luminal spaces, cytoplasmic vacuolization, hobnail cells
-psammoma bodies common
Px: assoc c myometrial and vascular invasion, and is often spread out of uterus at dx
- can be cured if there isn't any extrauterine spread (4/5 survival), otherwise will come back and kill pt; very aggressive
- not graded bc all are considered high grade
- need to look to see if there is stromal invasion to distinguish bwt low and high potential for malig
Cyto: papillae can break off on pap smear and show highly malig cells
IHC: (+) p53 (either strong and diffuse or completely negative ["null" phenotype]), p16 (strong, diffuse pos), ER/PR (1/2+, ER weak and patchy) CK7, CA125, very high Ki67, ER/PR (either ER+/PR+ or ER+/PR-, and can be weak and patchy), HER2/neu (in 1/3, usually lateral/basolateral with lack of staining in the apical portion of the cell membrane)
- negative: WT1 (but is pos in ovarian serous ca), CEA, CK20, HNF-1beta
- up to 90% of cases has p53 mutations, which correlates with abnormal p53 expression by IHC
- usually p53 has diffuse and strong nculear staining in >75% of tumor cells (only 10% of serous EACs show complete absence of p53 immunoreactivity due to nonsense mutations or homozygous gene deletion
- p53 mutation is an early event in serous carcinogenesis
Genes: TP53 mutations well characterized alteration in most cases
Tx: Therapies with HER2/neu now being investigated

Serous endometrial intraepithelial carcinoma (A) and serous endometrial carcinoma (C) show strong, diffuse nuclear immunoreactivity with p53 in most cases (B and D) (hematoxylin-eosin, original magnification x10 [A and C]; original magnifications34 [B] and x10 [D]).

Human epidermal growth factor receptor 2 (Her2) immunostain in serous endometrial carcinomas (A) showing a characteristic lateral/ basolateral membranous staining pattern (B) with lack of staining in the apical portion of cell membranes (hematoxylin-eosin, original magnification x20 [A]; Her2, original magnification x20 [B]).
Giant cell Carcinoma
Uncommon, has lots of bizarre nested GCs, lots of atypical mits
- IHC: (+) EMA, keratins
- neg: hPL, melCAM, CD146
- px: aggressive, usually assoc c other ca types
Hepatoid Carcinoma
Rare (few reported), post-menopausal c vag bleeding, assoc c other ca's (usually em-oid ca)
- IHC: (+) AFP (can monitor c serum AFP)
- px poor
Lymphoepithelioma-like Carcinoma
Rare, post-menopausal women, similar features as same ca in other sites (grps / sheets of polygonal cells c indistinct cell borders, vesicular nuclei c big nucleoli, mixed chronic inflam
- IHC: (+) EMA
- neg LCA
Transitional Cell Carcinoma
similar features as ca of urinary tract (papillae lined by transitional epithelium); usually in post-menopausal and assoc c other ca types
- mod to severe atypia; rare nuclear grooves, low mits
IHC: (+) CK7, p16 (diffuse), P63 (basal cells)
- neg CK20 (rare +), ER/PR
Carcinoma with Trophoblastic Differentiation
Rare, post-menopausal, inc serum hCG
- usually isolated chorioca or syncytiotrophoblasts mixed c em AC
- px perhaps better if only few syncytiotrophoblasts
Undifferentiated / dedifferentiated Carcinoma
Rare, 2 tumor subtypes, usually a mix of emdometriooid AC and medium-sized monotonous epithelial cells in solid pattern, but can have variety of other cell types and most have some necrosis
Micro: eccentric nucleo, vesicular chromatin
IHC: focal PanCK + (may be diffuse), focal EMA+, neg Pax8
Genes: 1/2 have MSI c MLH1 promoter methylation and MLH1 / PMS2 loss of expression
Dedifferentiated carcinoma
Mixed Tumors
Contains epithelial and mesenchymal parts, difficult to dx on bx
Adenofibroma
B9, uncommon, most in uterine corpus, sessile pedunculated papillary neoplasm c fibromatous stroma and b9 glands lined by columnar /cuboidal epithelium c em, ec or tubal differentiation, possibly sq diff or hyperplastic changes
- no stromal atypia or clustering around glands, low mits (<1 / 10 hpf)
- may harbor AC, or have fat, may recur
Adenomyoma
Uncommon, b9, well-circ, em-type glands in stroma c sm, occasionally fibrous
- glands have lots of space bwt them and are well-oriented
- smooth muscle (sm) has variable cellularity, up to 5 mits/10 hpf
- em-type adenomyoma has markedly irreg glands in stroma c lots of sm c minor fibrous part, CEA focally expressed sometimes,
Atypical polypoid adenomyoma (APA)
Polypoid mass in lower uterine segment in wormen of reproductive age, usually > 2cm
Disorganized hyperplastic glands in stroma c intersecting fascicles of sm or fibromuscular tissue, squamous morules, myofibromatous stroma, glands lined by cuboidal to columnar / pseudostratified epithelium and have variable size/shape, and of course may harbor AC (h-caldesmon to differentiate)
- (younger [<39yo]) pt presents c DUB, need long term fu c pt
Need to be completely excised or may recur
Atypical polypoid adenomyoma

Adenosarcoma
Usually in elderly (~60 yo), c benign epithelial and sarcomatous mesenchymal components
- ~ 5% of uterine sarcomas
Malignant stroma c b9 / hyperplastic epithlium, usually post-menopausal pts c uterine bleeding, MC from fundus
- need mod-marked atypia of stromal cells, periglandular cuffing, or >2 mits/10 hpf in stroma (should have at least 2 of these features)
- glands usually vary by size and shape and lined by prolif em / ec / sq / tubal / clear cell / secretory epithelium
- can see fat, sm cartilage, sex cord-like differentiation
- can have papillary fronds
- called sarcomatous overgrowth if pure sarcomatous area replaces >1/4 area of otherwise typical AC; aggressive (even if <1/4 usually)
IHC: Ki67 has periglandular cuffing, >1/5
Px: most important factors are myometrial invasion and sarcomatous overgrowth
Adenosarcoma - when >1/4 of the stromal component is pure sarcoma, the tumor is called adenosarcoma c sarcomatous overgrowth

Malignant mixed mullerian tumor (MMMT, carcinosarcoma)
Rare, biphasic high grade tumor with epithelium and stroma which can occur anywhere in female rep tract
- postmenopausal chicks c pelvic pain, ranges from 1-10 cm
- cartilage clinches the dx (? tho may be em ac c focal cartilagenous or osseous metaplasia; differentiate c atypia + mits)
- strong risk factor is pelvic radiation
- several theories exist as to how arises (collision theory, combination theory, composition theory, conversion theory [metaplastic carcinoma c sarcomatous part being made 2/2 inc aggressiveness])
May just have one or another of the elements:
-if both (stroma and epithelium) are b9: call it adenofibroma, adenomyoma
- just malig mesenchyme: adenosarcoma
- just malig epithelium: carcinofibroma
- Both malig components: carcinosarcoma (MMMT)
Micro: biphasic, c carcinoma and sarcomatous parts
Epithelial portion can be em, serous, clear cell, undifferentiated, squamous, while stroma can be homologous / heterologous
Homologous: (native to the tumor site) endometrial stromal sarcoma or leiomyosarcoma or undifferentiated sarcoma
Heterologous: (not native to the tumor site) muscle, cartilage, bone, fat
- common to see lymphangio invasion
- usually heterologous component more prominent in uterus (vs homologous component in cervix)
- mets to peritoneum can look different with papillae, psammoma bodies etc etc
IHC: (+) keratins (+ in carcinomatous and sarcomatous parts), EMA, p53, vimentin, fibronectin
- progressive loss of E-cadherin
- miR-200 overexpressed 2/2 rhabdoid differentiation (???)
Tx: TAHBSO if the LNs in pelvis suspicious, rads + chemo, recurrence recur in lungs n abd
Px: 1/3 alive at 5-years
- chondrosarcoma has slightly better outcome, rhabdomyosarcoma has worst outcome
MMMT

Carcinosarcoma

Pure Mesenchymal Neoplasias
arise in em stroma, sm, striated muscle, perivascular cells, bv, fat
Neoplasm arising from endometrial stroma
Highly cellular leiomyoma
Endometrial Stromal Nodule (ESN)
Shares features c endometrial stromal sarcoma (EMSS), but b9
Circumscribed c pushing margin and projections into adjacent myometrium up to 0.3 cm (possibly up to 0.6 cm; difficult to eval on bx), variably sized (1-20 cm, 7 cm avg), low mits
- cells are oval / round, rarely has hemorrhage
- BVs are predominantly arterioles c thick walls, neoplastic cells can whorl around BVs
- no vascular invasion, low mits (1-5 / 10 hpfs, can be up to 24), but not atypical mits
- IHC: (+) CD10
- neg: desmin (-/+), caldesmon (-/+), SMMS1 (-/+)
Highly cellular leiomyoma
slighly irreg contour, round / oval / spindly cells
- thick-walled bv's
Endometrial stromal nodule

Endometrial Stromal Sarcoma (EMSS)
Shares features c ESN, except invades vessels and / or myometrium, usually in rep age pts, may be single to multiple masses
very irreg contour that infiltrates myometrium, invades vessels
- can have worm-like pattern of invasion into myometrium
- uniform oval / round cells, or short spindle cells c arborizing vasculature c fine chromatin (HG can look similar to LG)
- BVs are predominantly arterioles c thick walls
Gross: can feel like a bag of worms 2/2 lots of vascular invasion
IHC: (+) CD10, actin, B-catenin overexpression, Ki67 (in proliferating stromal cells), ER/PR (diffuse)
- neg: desmin (-/+), H-caldesmon, SMMS1 (-/+)
-- HG vs LG tumors have diff IHC ... ? (HG is cyclin D1 (+) and CD10 neg, but can have HG areas within LG tumors)
- LG lesions notoriously positive for smooth muscle markers, causing diagnostic dilemma
- mits / Ki67 cannot really help to distinguish low from high grade (high-grade tumors are undifferentiated and do not look like normal stroma)
Genes: higher grade lesions assoc c t(10;17)(q22;p13)(YWHAE-FAM22) and while typical LG= EMSS has genes rearrangements in chromatin binding (JAZF1, SUZI2, PHF1, and EPC1)
-- high grade YWHAE translocation may be assoc c cyclin D1 expression and moderate CD117+, in which case they are almost always CD10 negative
Px: HG EMSS Tx is radical hysterectomy and have poor px
- LG EMSS have better px than leiomyosarcoma
- mets to ovaries, abdominopelvic cavity, lymph nodes (ESS one of the MC gyn sarcomas to met to pelvic LNs), omentum and lungs
low-grade endometrial stromal sarcoma

Undifferentiated Endometrial Sarcoma
- aka Poorly Differentiated Endometrial Sarcoma or Undifferentiated Uterine Sarcoma (UUS)
- does not have vascular pattern seen in EMSS; more a dx of exclusion
- cells round to spindle in mostly sheets, focal fascicles, post-menopausal pts, highly aggressive, invasion is confluent and destructive; has markedly enlarged nuclei, multinucleation and bizarre nuclei
- hard to say what it is or where it came from on histology
- IHC, should do keratins, EMA, desmin, CD45 to exclude other ca, CD10 (+) (can be expressed by other low or high gade lesions), may overexpress p53
- Gene: lack YWHAE-NUTM2 translocation
Endometrial Stromal Tumor (EST)
Low vs High-Grade
Low grade EST can be Endometrial Stromal Nodule or Low-grade endometrial stroma sarcoma (LG-ESS)
Low-Grade Endometrial Stromal Sarcoma
micro: monotonous sheets of small cells c ovoid to round nuclei, vesical chromatin, should only have small foci of invasion (usually well-circumscribed), spiraled arterioles common, ovoid bland cells, usually c lymphovascular invasion
Lymphovascular invasion (LVI) can help distinguish LG-ESS from an endometrial stromal nodule (ESN)
IHC: IFITM1 or CD10 (+) diffuse, ER/PR (+), var focal desmin (+), SMA (+)
- neg H-caldesmin (which would be + in smooth muscle tumors)
Genes: JAZF1-SUZ12
Px: recurrence in 1/3 (use hormonal tx)
A, Fragments of low-grade endometrial stromal sarcoma in an endometrial curettage specimen. The tumor cells are strongly and diffusely positive for CD10 immunostain (B), but also show diffuse cytokeratin AE1/AE3 (CK AE1/AE3) expression (C). Epithelial membrane antigen (EMA) immunostaining (D) is negative; note the internal positive control of detached epithelial fragment in the upper left corner. Desmin immunostaining is negative (not shown) (hematoxylin-eosin, original magnification x4 [A]; original magnification x4 [B through D]).
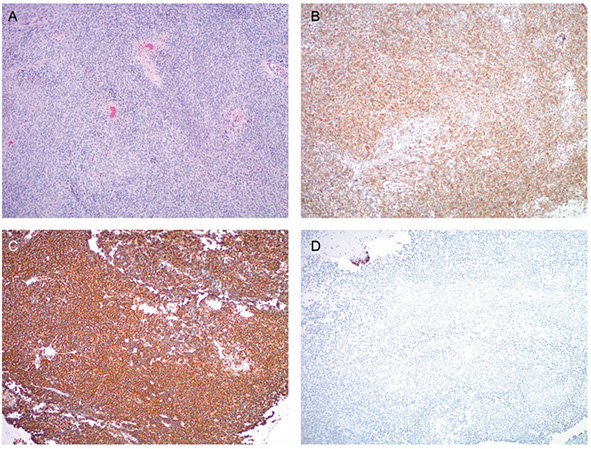
High-Grade Endometrial Stromal Sarcoma
Usually have diffuse Cyclin D1 positivity
- though must be careful bc uterine LMS can also be diffusely cyclin D1+
(+) BCOR
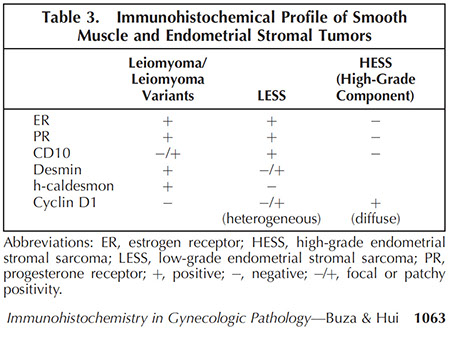
Mixed Endometrial Stromal / Smooth Muscle Tumors
- minor component must occupy >1/3 of lesion, similar to EM stromal tumors in behavior
Lesions arising from smooth muscle (usual type)
Leiomyoma (LM)
MC mesenchymal neoplasm of uterus, having bland spindle cells c cigar-shaped nuclei and mits up to 4 / 10 hpfs, can have wide variety of features
Hereditary Leiomyotosis Renal Cell Carcinoma
Assoc c RCC and fumarate hydratase abnormalities (in the citric acid cycle)
- have cutaneous leiomyomatosis and large uterine leiomyoma in younger pts
- assoc c type II papillary RCC
- nuclei look viral with cherry red appearance and nuclear clearing
Symplastic leiomyoma
Leiomyoma with lots of nuclear atypia
- has been compared to ancient schwannoma
- may be related to leiomyosarcoma
Symplastic leiomyoma

Submucosal Leiomyoma
- can be mistaken for em stromal lesion, having lots of edema, ulcerative necrosis, erosion, thrombosis
- IHC: desmin +, CD10 neg
Leiomyoma with Hyalin Necrosis
Hyalin (infarct-type) necrosis has necrotic area separated from tumor by hypocellular, hyalinized tissue band; can have hemorrhage but not inflammation around necrosis
- BVs usually have "ghost" outline in necrotic area, may mimic coagulative necrosis of leiomyosarcoma (which has hemorrhage too , but also inflam around necrosis not seen in leiomyoma)
Neurilemmoma-like Leiomyoma
- leiomyoma c Verocay bodies (like a schwannoma)
Mitotically Active Leiomyoma
- cellular leiomyoma c mits from 5-19 / 10 hpfs
- usually assoc c prolif phase, preg, hormone use (P)
- can have mild atypia / pleomorphism, have hemorrhagic areas, rare vascular invasion
- should consider inc fu in these pts
Leiomyoma with Hormone-Related Changes
Pts taking P can have LM that bleeding, mucinous change, edema, focal hypercellularity, mild pleomorphism, inc mits (esp around necrosis [must dinstinguish from coagulative necrosis])
- up to 1/3 LM in preg pts have degenerative pattern (may be termed apoplectic LM or hemorrhagic cellular LM) and can have cystic degeneration
- GnRH reduces the size of LM and can have apoplectic changes
Leiomyomas with Hyalin and Hydropic Degeneration
Hyalinization common in LM in post-menopausal (PM), having well or poorly circumscribed edema, prominent BVs of variable caliber, and granular eosinophilic material, possibly cystic change, c nodular pattern of neoplastic cells around vessels
- can rarely have vascular invasion
Cellular and Highly Cellular Leiomyoma
- difference in cellularity from surrounding myometrium is obvious, but in highly cellular LM you start to get concerned about EMSS
- grossly they are tan and softer than regular leiomyomas
- have thick-walled BVs throughout the tumor
- highly cellular LM can have rough borders, and clefting w/wo endothelial lining, have diffuse pattern in the center and fascicular pattern at periphery of lesion, c mild to focal bizarre atypia, and thick BV
- IHC: (+) desmin, caldesmin, SMMS-1, focal +/- CD10

Leiomyoma with Heterologous Elements
have variable fat (lipoleiomyoma), usually in corpus, tho can be in cervix
- can rarely have brown fat, bone, cartilage, sk m
Leiomyoma with Vascular Intrusion
- can be LM c vascular intrusion if intrusion found in tumor or microscopic intravascular leiomyomatosis if seen outside tumor
- have little risk of recurrence or mets, but should fu more and possibly do imaging
Tumorlet
Small LM, either focal or multifocal, cells epithelioid or spindle shaped in cords / trabeculae, no clinical sig
Smooth Muscle Tumors of Uncertain Malignant Potential (STUMP)
Features preclude straightforward dx
- Smooth muscle Tumor of Uncertain Malignant Potential (STUMP) includes: atypical LM c low risk of recurrence, atypical LM but limited experience, SM tumor of low malignant potential but limited experience
- tumors bizarre shapes, dark nuclei c smudged chromatin, cytoplasmic pseudoinclusions either focally, multifocal, or diffuse, usually low mits, sometimes atypical mits
- p16 positivity may be more assoc c recurrence; rare cells can be + for Ki67
Leiomyosarcoma
1-2% of uterine malignancies (but MCC uterine sarcoma); 54 yo avg
- abnormal vag bleeding
Gross: fleshy invasive tumor c hemorrhage and >5cm, but no multiple tumors
Micro: must have 2 of 3 (from most to least important):
1) coagulative necrosis
- abrupt necrosis with ghosts of tumor cells, usually no zone of intervening granulomatous reaction
2) mits >10 / 10 hpfs,
3) diffuse atypia
- hypercellularity also important
- atypical cells have irreg nuclear contours, uneven chromatin, variably prominent nucleoli
- hypercellular pleomorphic spindle cells that may resemble sm muscle
- infiltrative border, and invades vessels, can rarely have osteoclast-oid GCs or other sarcoma (liposarc, rhado, osteosarc)
- commonly see coagulative tumor cell necrosis (important in distinguishing from b9)
- should have >1 mits per hpf in 10 hpf's, which are atypical
IHC: (+) SMA, myosin, desmin ER, PR, EMA, h-caldesmon, keratin (CAM5.2), p53, p16, histone deacetylase 8 (HDCA8), high Ki67
- negative CD44v3, CD10 (var), CD99, inhibin, calretinin, Melan-A, CK's (var)
Special stains may be helpful, showing a honeycomb appearance around the necrotic cells with reticulin staining, and lack of blue collagen fibrosis with trichrome staining
Genes: up to 1/2 have p53 mutation, p16 overexpression
Tx: TAH, debulking
- chemo / rads can be helpful
Px: Aggressive, 40% 5 yr survival, 10% if anaplastic, worse if extends out of uterus
- should be graded (1-3) using the French Federation of Cancer Centers Sarcoma Group (FNCLCC??)


Leiomyosarcoma
Leiomyosarcoma - necrosis
Epithelioid smooth muscle neoplasms
Uncommon; Round / polygonal sm cells c red-clear cytoplasm, usually has lots of atypia, inc mits, coagulative necrosis
- IHC: usually stain for keratins, not as much sm markers
Myxoid smooth muscle neoplasms
Myxoid LM exists, but do not dx in curettage (limited to hysterectomy specimen bc must do exam of margins), are well circumscribed c low mits (up to 5 mits / 10 hpfs)
Myxoid LM-sarcoma is invasive and have more atypia c coagulative necrosis and any degree of mits
Uterine tumors resembling ovarian sex cord tumors (UTROSCT)
Uncommon, has prominent sex cord differentiation
- arises in uterine cavity, but can protrude through cervical os, variable age range, grossly lobulated and can look like LM from whorls, range from 4-10 cm
micro: arranged in nests, cords, trabeculae, tubules, papillae, looking like Sertoli-Leydig cell tumor of ovary in >1/2 of tumor
- can have nuclear grooves
IHC: must share at least 2 immunomarkers as sex-cord stromal tumors (CD99, inhibin, calretinin [most sensitive], melan-A), also (+) SMA and desmin (var), ER/PR, CD10 (focal), , CD10 (var, focal pos), CK's
- neg: h-caldesmon
px: more b9 if not infiltrative and no BV invasion
UTROSCT

Rhabdomyosarcoma
usually a component of MMMT or adenosarcoma, rarely pure, but can occur in 2 forms:
1) embryonal rhabdomyosarcoma - as sarcoma botyroides in younger pts c cervicovaginalpolyps
- has round, oval, polygonal cells in sheets and broad fascicles or as scattered cells, can see b9 entrapped glands in polyps
- botryoid variant has cambium layer c hypercellular zone of undifferentiated round / spindle cells beneath epithelial surface
- IHC: (+) myogenein, myoD1
- better px if arises in cervix of younger pts, slightly older pts can have DICER-1 mutation; must alert physician to possible presence of lung tumors or ovarian sex cord-stromal tumors
2) pleomorphic rhabdomyosarcoma - pm pts
- micro: large, round, bizarre polygonal to spindle cells in fascicles, sheets, hemangiopericytic or storiform patterns, lots of rhabdomyoblasts
IHC: intermediate bwt LM-sarcoma and rhabdomyosarcoma
- px: very aggressive, presents at advanced stage
- should sample well to exclude MMMT
Hemangioma
rare, b9, can bee seen on US, can cause bleeding, can be polypod c either cavernous or capillary formation
Angiosarcoma
usually PM, though can be younger pts; Interanastomosing vascular channerls lined by atypical endothelial cells c epithelioid or spindly appearance
- solid areas, tufting, papillae, necoris, inc mits, cystic change and bleeding can be seen
- IHC: (+) CD31/34, factor VIII, keratin (if has epithelioid diff)
- px: very aggressive, most die within 1 yr
Lipoma
very rare, made only of fat cells, can be as large as 17 cm
- should examen lesion thoroughly
Liposarcoma
very rare, usually in PM, can be in uterine body or cervix
Adenomatoid tumor of uterus


Adenomatoid tumor
Small, most are subserosal, so unlikely to be sampled in curettage
- can be nodular or ill-defined
Micro: have tubular, cystic, or papillary structures c slit-like spaces lined by flat to cuboidal cells wo mits; can have rare atypia
- spaces usually have hyaluronic acid, which can stain c acid mucin stains
IHC : (+) calretinin, CK, vimentin, WT-1
- calretinin would be the best stain to separate from smooth muscle tumors or epithelial tumors
Perivascular epithelioid cell neoplasm (PEComa)
Uncommon, in uterus or cervix, made of epithelioid or spindly cells c red to clear cytoplasm in fascicular, nested or solid pattern around BVs, which can be prominent and delicate or thicker
- most have low to mod atypia / cellularity / and mits
- can have atypical mits, MNGCs, spider-like cells, coagulative tumor necrosis, BV invasion, infiltrative margins
- can be difficult to differentiate form epithelioid sm neoplasm (which has less vessels)
- IHC: (+) HMB45, Melan A, SMA, desmin
- prognostic features stratified according to tumor size, infiltration, nuclear grade, cellularity, mits, necrosis, vascular invasion
Lymphoma
Although lymphoma can disseminate to gyn in up to 1/3, primary lymphomas are rare (1/100 extranodal non-Hodgkin lymphomas), usually in cervix with barrel lesion, rarely infiltrates, sclerosis common, B-cell lymphoma MC, but other types seen too
- must differentiate from florid reactive lymphoid hyperplasia, leiomyoma c extensive lymphoid infiltration, severe chronic endometritis, follicular cervicitis, PNET/NEC, (granulocytic) sarcoma
- must do full workup (bmbx) if it is lymphoma
Neuroendocrine Tumors of the Cervix
up to 1/50 cervical neoplasias
- HPV assoc c atypical carcinoid, SCNEC and LCNEC
Typical and Atypical Carcinoid
Resembles counterparts in GI and lung
Typical carcinoid has small, uniform cells c finely granular chromatin in chords, trabeculae or nests, no mits
Atypical carcinoid has inc cellularity, nuclear pleomorphism and inc mits 5-10 / 10 hpf) and possibly necrosis
IHC: (+) SYN, CD56, CHR
Px: atypical carcinoid more likely to met probably (not much experience)
Small and Large Cell Neuroendocrine Carcinoma
- resemble lung counterpart, can have paraneoplastic Cishings, carcinoid, hypoglycemia, SIADH
- early nodal and distant mets, worse than SCC
- usually seen c other cervical ca subtypes
Small Cell Neuroendocrine Carcinoma (SCNEC) has small dark cells c finely granular nuclei c no nucleoli, lots o mits, necrosis common
IHC: some cses may be SYN, CHR CD56 negative! and TTF-1 + in up to 1/3; CK can help distinguish from lymphoma
Large Cell Neuroendocrine Carcinoma (LCNEC) has larger cells than SCNEC), usually c more cytoplasm, vesicular nuclei, lots of mits and apoptotic bodies, and comedo-like or geographic necrosis
Primitive Neuroectodermal Tumors
Ewing / peripheral PNET tumor family and those that look like central-type PNETs like medulloblastoma or neuroblastoma
- IHC: (+) SYN, CD99, neurofilament protein
- neg: keratins, EMA,
- should do PCR if tumor negative for CK but express SYN and neurofilament to exclude Cr 22 rearrangement, which can alter tx
Melanocytic lesions are rare, but can have incidental melanosis, blue cell nevi, malignant melanoma; (+) S100 and HMB45, MART1
Inflammatory Myofibroblastic Tumor
Uncommon in uterus, has spindly, stellate, or ganglion-like cells c minimal cytoplasm, variable myxoid stroma, lymph infiltrate, low mits
- originally thought to be a pseudotumor, but rare cases met or relapse, and has ALK receptor gene translocation at cr 2p23
- IHC: (+) all have 1/4-1/2 of cells + for ALK
Germ Cell Tumors
Very rare, yolk sac tumor most common, AFP+, CK7 neg, EMA neg
- primary teratomas can be mature or immature (look for immature neuroepithelium)
Gestational Trophoblastic Disease
- encompasses molar dz (hydatifdiiorm mole, persistent gestational trophoblastic dz, invasive moles) and trophoblastic tissue neoplasms (choriocarcinoma, placental site trophoblastic tumors, epithelioid trophoblastic tumors)
Hydatidiform Mole
References
1. Buza N. Immunohistochemistry in the Gynecologic Tract. Arch Pathol Lab Med 2017; 141:1052-1071
2. Yang E, Mutter G. Biomarker resolutaion of uterine smooth muscle tumor necrosis as benign vs malignant. Mod Pathol 2015, 28, 830-835
3. Wang H. A Timely Update of Immunohistochemistry and Molecular Classification in the Diagnosis and Risk Assessment of Endometrial Carcinomas. Arch Pathol Lab Med 2021.
