refer to Lung Neoplastic
Respiratory Cytology
Sampling methods
- Sputum
- Bronchoscopy
- FNA
Benign changes
- Squamous metaplasia / Reactive cells
- Radiation and chemotherapy
- Reserve cell hyperplasia
Contaminants and non-cellular stuff
- Vegetable matter and Pollen
- Corpora amylacea
- Charcot-Leyden crystals
- Creola bodies
- Curschmann spirals
- Ferruginous bodies
- Psammoma bodies
Bugs (Microbiology)
Squamous cell carcinoma
Brochioalveolar Carcinoma (BAC)
Large Cell Carcinoma
Carcinoid tumor
Small Cell Carcinoma
Adenoid-cystic carcinoma
Malignant mesothelioma
Adenocarcinoma
- Mucinous adenocarcinoma
Pulmonary Hamartoma
- PH with myxoid stroma
Clear cell ("sugar") tumor
Epithelioid Angiosarcoma (EAS)
Sampling methods
-- see Lung
Sputum
Healthy pts usually don't produce sputum, but this mucus soln contains cells and non-cellular stuff
- must have macrophages in sample to be satisfactory
Pt should rinse mouth first to remove contaminants (food, bacteria) and the best time to take it is first thing in the morning with deep coughs for 3 consecutive days
- can give a nebulizer (has H20, sodium carbonate, and glycerin) to help induce sputum production
Pros: Non-invasive, no complications, low cost and fair accuracy (80%), good to screen for lung ca
Cons: Cannot localize tumors, diagnostic cells may be few and degenerated (from the time it takes to travel up the respiratory tract), doesn't work if blockage in resp tract, rarely works with single nodules
Bronchoscopy
After application of a local anesthetic the bronchoscope is inserted through the nose and a specimen can be taken using a bronchial brushing, bronchial washing (taken after the brushing usually), or by transbronchial FNA
- BAL esp good at detecting opportunistic infections
- can be done to detect cancer within individual bronchial segments
FNA
Transthoracic FNA goes through the body cavity rather than through a brochoscope, which may be esp useful with small, peripheral tumors
Indications: solitary lung nodules in pts c negative cytology, differentiating new primary vs mets in a pt c known cancer, multiple nodules in a pt c unknown primary, to differentiate infx vs ca
Pros
Cheaper, less risky and faster than surgery, high accuracy, detects infx
Cons
Hemopytsis, pneumothorax
Contraindications: pt on anticoagulants, COPD, severe pulmonary HTN, severe emphysema, uncontrolled cough, uncooperative / unconscious pts, vascular lesions (bleeding risk)
Normal histology and cytology
Refer to Respiratory - Anatomy and Histology
Benign changes
Degenrated squames
Nuclei are small pyknotic and condensed and not very pleomorphic (as in SCC)
Squamous metaplasia / Reactive cells
Reactive condition (seen often in fungal conditions and next to a stoma) with replacement of ciliated columnar cells by squamous cells - not necessarily premalignant
Micro: smaller cell size c inc N:C,
- as it becomes more atypical, cells tend to become single with more nuclear atypia
Reactive cells appear enlarged with inc chromatin granularity, thick and irregular nuclear borders, prominent (sometimes irregular and multiple) nucleoli
- can be very difficult in respiratory tract to tell reactive from carcinoma, terminal bar and cilia can help
Radiation and chemotherapy
Radiation can cause cytoplasmic enlargement, vacuolization, spider projections with nuclear enlargement, multinucleation
Chemotherapy causes nuclear enlargement with nuclear smudging and cytoplasmic enlargement with 2-tone cytoplasm
Reserve cell hyperplasia
Reserve cell layer becomes hypertrophic and stratified in response to toxins (cigarette smoke)
-when overlying cells are shed the underlying reserve cells mature to metaplastic squames with cell flattening, karyopyknosis and keratin production
Micro: small fragments of tightly cohesive reserve cells c hyperchromatic nuclei, nuclear molding and a thin rim of basophilic cytoplasm, slightly larger than normal reserve cells
DDx: small cell carcinoma (looks similar, but has necrosis)
- lymphoma, non-K SCCA
Contaminants and non-cellular stuff
Alernaria spp
Airborne fungus considered a contaminant
Plant cells (Vegetable matter), pollen
Floated in there 5$ if you can name the correct vegetable / plant
Corpora amylacea
Large blobs in the lung that are not calcified (though they are in other organ systems); no known significance
Charcot-Leyden crystals
Squarish, orangy crystals that are formed when eos degenerate and are assoc c severe asthma
- thus are formed from the lysophospholipase of eosinophils
Creola bodies
3D sphere of reactive ciliated bronchial epithelial cells assoc c asthma and other chronic lung dz
- named after a patient in whom a false positive diagnosis of adenocarcinoma was made
Curschmann spirals
Nonspecific; should not be formally mentioned to avoid confusion
- may be assoc c asthmatic bronchitis
Ferruginous bodies
Sometimes called "asbestos bodies" are golden-black dumbbell-shaped thingies
Protein
May be suggestive of amyloidosis or alveolar proteinosis
Psammoma bodies
Circular calcifications assoc c malignant neoplasms, such as adenoca and mesothelioma and metastatic malignancies (ovarian and thyroid ca) but also with benign diseases like TB
Bugs - see Microbiology
Standardized Terms / Nomenclature for Resp Cytology (Diagn Cytopathol, 2016; 44:399-409)

SEER Data for Lung Ca 2010-2014

Clara cells - nonciliated cuboidal-columnar cells that line terminal bronchioles
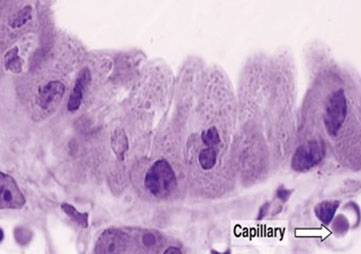
Reactive cells



Vege matter


<--- Alternaria
Charcot-Leyden crystals
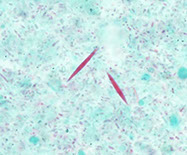
Creola body



Curschmann spirals

Cryptococcus

Strongyloides
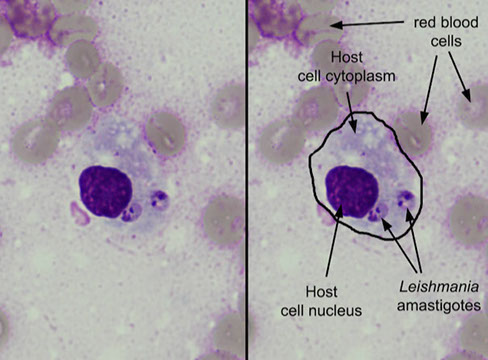

Adenocarcinoma
Originates more distally in subsegmental or distal bronchi; more easily detectable with radiology; more often found in females
- rarely dx'd on cytology unless comes from mucus gland or larger bronchi
Mciro: Smooth cannon ball-like edge of cell clusters, or honey-comb sheets, papillae, acini; Nuclei are eccentric and round with finely granular chromatic and big nucleoli; cytoplasm is abundant, finely vacuolated (foamy) sometimes with large secretory vacuoles and arranged in small cell clusters or as single cells; (+) mucin vacuoles,
(+) PAS
- adenoca has finer chromatin, more mucin and acinar formation, no keratinization and lighter cytoplasm than SCC
Genes: EGFR and ALK testing are done on any lung adenocarcinoma and any mixed lung tumors with an adenocarcinoma component
- EGFR somatic mutations in exons 18 to 21 of the tyrosine kinase domain of EGFR occur in ~10% of Western and ~1/2 of Asian patients with pulmonary adenoca
- KRAS occurs in adenoca assoc c smoking (poor px)
- EGFR and KRAS mutations are mutually exclusive
Do not perform EGFR and ALK testing on pure SCC or small cell ca
Drugs that target EGFR include:
Erlotinib (Tarceva®), Afatinib (Gilotrif®), gefitinib
EGFR mutations more common in women and people who haven’t smoked
- gefitinib is an antagonist of EGFR-TK, pts c L858R mutation in EGFR are potential candidates to get targeted tx c gefitinib
Drugs that target ALK include:
Crizotinib (Xalkori®), Ceritinib
ALK mutations most often seen in non-smokers (or light smokers)
Drugs that target VEGF include:
Bevacizumab (Avastin®)
Mucinous adenocarcinoma
spread via airways and have satellite lesions
- can have extracellular mucin in lepidic pattern
- has KRAS mutation
Imaging: Appears solid on CT, may resemble lobar pneumonia if entire lung consolidated`
IHC: (+) TTF-1, CK20, CK7 negative (thus often confused c metastatic colon ca!)
- may be TTF-1 negative and CK7/20 (+)
Micro: honeycomb sheets c eccentric nuclei, lots of foamy cytoplasm with mucin vacuoles
Adenocarcinoma In Situ
Can have flower-petal appearance
Pitfalls
Pneumonia
Reactive pneumocytes c ARDS
Adenocarcinoma





Mucinous adenocarcinoma of lung

Organizing pneumonia
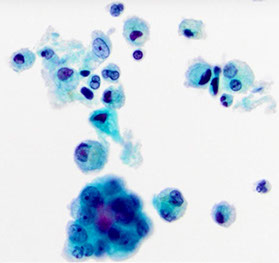
Reactive pneumocytes (pt c ARDS)



CIS
Slight ASM
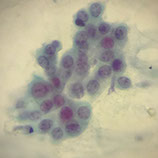
Marked ASM


Moderate ASM





Small cell ca



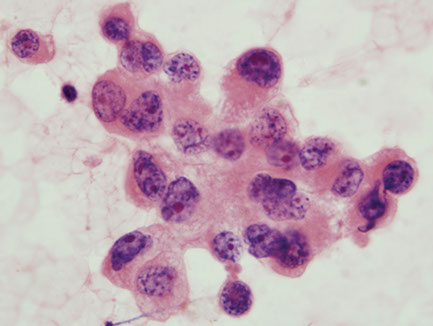
Large cell carcinoma
Squamous Metaplasia / Dysplasia (to CIS)
Regular metaplasia
Cells about same size; nuclei c same size c regular N/C; fine powdery normochromatic chromatin c rare chromocenters; basophilic cytoplasm; cells aggregated, but may be single
Slightly atypical metaplasia
Cells can vary in size; slight variation in nuclei size c slightly variable N/C; fine powdery chromatin c rare clusters of nuclear material near nuclear membrane; may have acidophilic cytoplasm; can be in sheets or single cells
Moderate Atypical Squamous Metaplasia (ASM)
Moderate variation in cell size; significant nuclear pleomorphism c moderate N/C variation; sometimes has fine powdery chromatin, but lots of nuclear clumping present; acidophilic cytoplasm predominates; sometimes in sheets, sometimes single cells
Marked ASM
Marked cellular and nuclear pleomorphism; coarse nuclear material; extreme variations in N/C ratio; small, sometimes acidophilic nucleoli present; acidophilic cytoplasm predominates; single cells predominate
Carcinoma in situ
Marked cellular / nuclear pleomorphism (up to double the size in marked ASM!), single cells present but clusters more common than in invasive ca; large globs of chromatin present in nucleoli, not necessarily near membrane; N/C has wide variations (very low to very high) and is very pleomorphic; cannabalism and multinucleation present; acidophilic cytoplasm predominates
Squamous cell carcinoma
MCC lung ca and cancer death (~1/3%); usually comes from larger bronchi, thus more central
- assoc c smoking
- tends to invade bronchial wall and cause obstructive pneumonia; also forms necrotic cavities
- vascularity of the lungs facilitate mets
Several different subtypes (papillary, clear cell, small cell) exist
Cavitary lesions assoc c freq ghost cells and necrosis
Focus on keratinization, Keratin pearls, intercellular bridges
IHC: p40 superior to p63 to confirm dx
DDx: should include mets, other types of lung / resp ca, contaminants
Keratinizing (Well-Differentiated) SCC
Orange cytoplasm (from keratin) - can be basophilic in brushings; lots of cellular and nuclear pleomorphism, may see spindle cells, tadpole cells, cannibalized cells, multinucleation; great variation in nuclear pigmentation (hyperchromatic to pyknotic) and chromatin patterns (bland to coarse and irregular); may see nuclear angulation; can be single cells or in clusters; nucleoli are infrequent, large and irregular; lots of variation in N/C - can even see lots of anucleate cells; necrotic debris and degenerating malignant cells usually there
Poorly differentiated squamous (epidermoid) ca
Cytoplasm is basophilic and scant or absent; nuclei are very large and hyperchromatic due to coarse irregular chromatin (may be hypochromatic if very undifferentiated); large irregular single or clustered, elongated cells; rarely keratinized cytoplasm; can see cannibalism; may or may not have nucleoli
- in sputums can see some irregular vacuolated cells that may look like adenoca, but should be called "undifferentiated large cell ca"; chromatin is more fine in adenoca and more coarse in uSCC [2] and cytoplasm is denser in SCC; small amts of intracellular mucin can be seen in SCC! (tumor may also be adenosquamous)
- appears pallisading in basaloid variant
BronchioAlveolar Carcinoma (BAC)
Well-differentiated adenoca that originates peripherally from terminal bronchioles or alveolar walls or old scars (scar ca)
- tumor cells grow along the alveoli (lepidiform?) and replace the normal resp epithelium and dec the respiratory capacity
- continued prolif of tumor cells leads to formation of papillary fronds
Micro: + grooves and nuclear pseudoinclusions; uniform cells c pale clear nuclei;
Undifferentiated large cells carcinoma
Shows no columnar or squamous cell features
- may be rediagnosed 2/2 tissue sections showing features
Large nuclei and nucleoli, which can be multiple and irregular with thick irregular nuclear membrane, although the nucleus is usually roundish; chromatin is clumped and irregular; cytoplasm is variable;
cells arranged in irregular groups
Carcinoid tumor
~5% of all bronchial tumors; can be typical or atypical based on mitotic index; atypical carcinoid lies bwt typical carcinoma and small cell ca
- arises from Kulchitsky cells (same as small cell carcinoma), usually in the large bronchi and protrudes into and causes obstruction of the main bronchi's lumen
- usually discovered by radiology or brushing
Micro: (typical) loose sheets or single cells c uniform cuboidal or elongated cells c abundant granular ("salt n peppa") basophilic cytoplasm without necrosis; nuclei are uniform and can be central or eccentric; small nucleoli are usually present; forms rosettes, see branching capillaries, few mits
- spindle cells in carcinoid tumors are MC from the periphery, as opposed to the more common central carcinoid tumors
(atypical) looser rosettes with slightly larger cells, perhaps with a little bit of molding; same salt n peppa chromatin, mit index slightly higher than typical (up to 10%), cells a little more pleomorphic and can see minor amts of necrosis
IHC: 1/3 of atypical carcinoid (+) for TTF-1
- usually neg for TTF-1
Px: 90% 5-yr survival, 10% have mets
- favorable patterns are pseudoglandular growth pattern, papillary growth pattern or palisading
Large cell carcinoma
5-10% of all lung ca., dx of exclusion
- peripheral (except basaloid subtype)
- mix of squamous and adenocarcinoma (?)
Highly anaplastic undifferentiated tumor with multiple subtypes
Micro: clusters or single cells c irregular nuclei, multiple prominent nucleoli, feathery cytoplasm, prominent chromatin clearing, inc mits
Makes B-hCG (?)
Px: Poor; not very responsive to chemo - removed surgically
Important variant: Large cell neuroendocrine carcinoma (trabecular or palisading)
Small cell ca.
Comprises up to 1/4 of all primary lung ca
- usually develops in the large bronchi (central)
Micro: Small cells (isolated, in chains and clusters) - about the size of lymphocytes
Nuclear molding and variable hyperchromasia (from moderate to opaque); chromatin is coarser than adenoca and finer than carcinoid, and is evenly distributed and powdery; nuclei round to slighlt oval and freq irreg c rounded sides - smudging of nuclei is also characteristic
Scant cytoplasm; no nucleoli, nuclear debris and crush artifact in background
LOTS O MITS (mit index usually ~80%)
- no lymphogranular bodies (LGBs)
IHC: (+) TTF-1
Px: Highly malignant, long-term survival is rare (avg survival of 1 yr after dx)
- early lymphatic and hematogenous mets
Adenoid-cystic carcinoma
aka cylindroma; derived from bronchial mucus gland; a rare tumor that is similar to the same tumor of the salivary glands
Usually found centrally in the major bronchi and trachea and dx'd c bronchial brushings or transbronchial needle aspiration
Micro: small uniform basaloid cells surrounding a core of homogenous material
Px: More aggressive than carcinoid, with mets to LNs and lung parenchyma
SCC


WHO 2015

Carcinoid
Carcinoid - uniform cells


Carcinoid - salt and peppa
Atypical carcinoid

Large Cell NeuroEndocrine Carcinoma (LCNEC)
Architecture:
• More cohesive sheets or clusters of cells
• May show rosette formation or nuclear palisading
Cellular features:
• Medium to large sized cells
• Round to oval nuclei
• High N/C ratio, moderate amounts of cytoplasm
Nucleus/chromatin:
• Open, clumpy chromatin
• Often prominent nucleoli
Other:
• Frequent mitoses
• Necrotic background
• Less nuclear molding
• Crush artifact (smears)
LCNEC


Malignant mesothelioma
cellular with large cells, nucleoli, high N:C ratio
<2% of all malignant effusions
Pleura > peritoneum > pericardium
80% of cases linked to asbestos exposure
• Latency of decades
Incidence USA peak: 1990’s
• Decline in the USA, continues to be a health issue worldwide
Common symptoms: chest pain, shortness
of breath
Unilateral pleural effusion
Gross appearance of effusion: consistency
and color of honey
Definitive diagnosis generally requires evidence of invasion into skeletal muscle/adipose tissue on pleural biopsy
•Cannot prove invasion on cytologic effusion specimen!
3 main types:
1) Epithelioid: most common; most effusions
2) Biphasic
3) Sarcomatoid: rarely exfoliates
2 main patterns:
1) Large clusters with scalloped borders (“mulberry clusters”)
- “Windows” and “lacy skirts” like normal mesothelial cells
- Clusters of >20-40 cells are indicative of malignancy
2) Numerous dyshesive cells
- “Mesothelial morphology” but with severe cytologic atypia
- Diagnostically challenging
Often need ancillary studies.
Genes: Homozygous 9p21 deletion has 100% specificity
• p16/CDKN2A tumor suppressor gene
- ~80% of mesotheliomas have some kind of alteration to the BAP-1 gene, which correlates c a loss of IHC expression
Mesothelioma


Malignant Mesothelioma
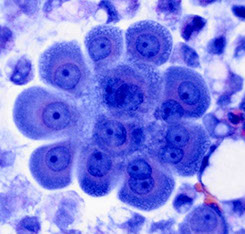

Pulmonary Hamartoma
MCC benign tumor of the lungs; relatively uncommon
- asymmptomatic
Micro: fragments of benign ciliated respiratory epithelium; irregularly shaped fragments of cartilage matrix with oval to elongated vesicular nuclei; adipocytes, bland spindle cells, fibromyxoid stroma
Tx: excision is curative
PH with myxoid stroma
Benign glandular cells
Immature fibromyxoid matrix and bland spindle cells
Mature cartilage with chondrocytes in lacunae
Adipocytes
Clear cell ("sugar") Tumor
extremely rare; can occur at any age; peripheral; asx
- come from perivascular epitheliod cells
Micro: bland coboidal to spindled cells; lots of cytoplasmic glycogen clearing
IHC: (+) HMB-45 / Melan-A
- negative CKs and CEA
Epithelioid Angiosarcoma (EAS)
Micro: large polygonal cells c vesicular chromatin, irregular nuclear membranes, prominent nucleoli
IHC: (+) CD34, CKs (in 1/3)
Pulmonary Hamartoma with myxoid stroma


References
1. Lecture notes, the U
2. Cibas Cytology, ch 2 Respiratory Tract, 3rd ed
