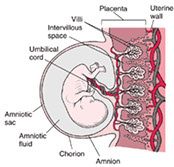
Placenta and Pregnancy
Placenta Gross
Placenta histology
IntraUterine Growth Restriction (IUGR)
Necrotizing funisitis
Syphilis
CMV
Parvovirus B19
Villitis of Unknown Etiology (VUE)
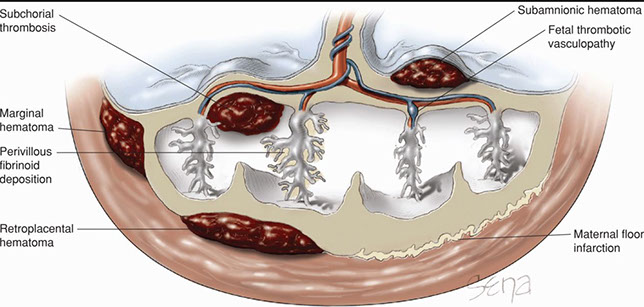
Maternal Floor Infarct (MFI)
Retroplacental hemorrhage
Chorangioma
Chorangiosis
Decidual vasculopathy
Vacuolated amnionic epithelial changes
Trophoblastic tumors
- Exaggerated Placental Site
- Placental Site Nodule
- Placental site trophoblastic tumor (PSTT)
- Epithelioid Trophoblastic Tumor
- Choriocarcinoma
Molar Pregnancy
-
Complete Hydatidiform Mole (CHM)
- Early CHM, Invasive CHM
- Partial hydatidiform mole (PHM)
Hydropic Abortus
Early abortus (EA) c trophoblastic hyperplasia
Abnormal villous morphology (AVM)
Androgenic / biparental mosaic conceptions (MOS), some with CHM
Metastasis to Uterus
Placenta Gross
Cord
35-75 cm at term, 55 cm avg (can tell long [from accidents, knots, proapse], but not really short [oligohydramnios, body wall defects, arthrogyropsis]), 2 umbilical arteries carrying de-oxygenated blood from fetus
- can insert velamentous (in membranes), vasa previa (if inserts over the cervical os), furcate
- knots (not always significant), look for dilated vessels on one side and
- coiling index: 0.2 cm - measure 10 cm and count twists, or just subjectively say it looks more twisted (hypercoiled)
- right twist less common?
- should do US on GI tract if single umblical artery
other cord problems: nuchal cord, torsion, stricture, marginal insertion (aka "battledore placenta" - not always significant), hematoma, iatrogenic puncture (look for hx of cordocentesis)
Membranes
- cover th fetal surface of the disk
- point of rupture (can help to know where the placenta was implanted in the uterus)
get bubbly and tall amniotic epithelium when meconium present
- artery crosses vein, in monochor twins may have A-A, V-V, A-V (last most sig in TTT)
- amniotic bands??
- can get amputation of extremites
Amnion nodosum - white/yellow dots that dont rub off, most apparent in oligohydramnios / Potts syndrome
- acute chrioamnionitis - icky yellow
Meconium staining - icky green; from fetal intestinal contents; passage prior to birth assoc c inc GA; timing innacurate and multiple episodes can occur - not able to really say if it means anything, physiologic response to birth - brown pigment fades under fluorescent lights (ie when left out on trays... look for the study...) - though meconium is vasoactive, causes cytokine release and vasoconstriction (but cant tell these unless have vascular muscle necrosis [meconium has to be there at least 15 hours, arteries affected 1st])
-- fetal inflam response: umbilical vasculitis, assoc c later CP esp in small infants; fetal IL-6 inc in these infants
Villi
"basal plate" of placenta that attaches to uterine wall, c infarcts and hemorrhages
- marked histo changes in villus morphoogy over inc GA
- size and stroma content dec as branching occurs
- inc % of area occupid by vessels
- syncytiotrophoblast nuclei aggregate c vasculosyncytial membranes
- Paleness and edema ssoc c syphilis
- infarction - white fibrous tissue that is usually MC on periphery of placenta since edges are usually more hypoxic
- reduced utero-placental blood flow - syncytiotrophoblast most sensitive, causing aggregation and smudging of nuclei; poor growth of placenta c reduced weight, villi become small from excessive branching
Decidua - plasma cells?
- implantation site vessels - intermed trophoblasts invade uterine arterioles and destroy muscle nd elastica; waves of invasion in first nd second trimesters; "physiologic remodeling"
- changes in pre-eclampsia - atherosis (foamy macrophages)
Shape variations:
bilobed
succenturiate
circumvallate (assoc c growth restriction and preterm labor - has a little pocket that you should be able to stick a probe in)
circummarginate
membranacea
(though they are more or less ameboid)
Fetal surface
- shiny, smooth, flat,
- cord insertion c branching vessels (A over V)
- chorioamnionitis, meconium



PAS?






Placenta histology
Earliest trophoblastic stage if a cell recognizable by the 12 to 16 cell stage as the outermost cell mass of the morula
Later flattens and forms embryoblast in the blastocyst stage
- invades the endometrial lining by the end of the 1st WGA
- lacunae form within the syncytiotrophoblastic layer as continues to invade into endometrium
Placental anatomy established by 2nd trimester, but continues to undergo changes
- the decidua comes from the parent, the chorion and amnion comes from the baby
-- small potential space bwt the amnion and chorion come from the remnant of the extraembryonic coelom
- trophoblastic cells of the chorion have variable sizes and shapes and are occasionally multinucleated
Retroplacental hemorrhage
- Premature separation
- blood acuumulates begind placenta
- assoc c pre-eclampsia, trauma, infx, smoking, cocaine
-extent of lesion (early lesions hard to dx)
MC findings in placentas:
- Preterm: ischemia and chorioamnionitis
- Term: ischemia nd meconium changes
- Post term: meconium changes and ischemia
Monochorionic placentas are identical, dichorionic can be anything
- mono mono dangerous bc cords usually get crossed
Chorangiosis ... other lesions ???
PreTerm Labor
- MCC is ascending chorioamnionitis
- circumvallate placenta is a cause of PTL and IUGR (but is actually caused by chronic peripheral abruption and fetal hypoxia)


Hematomas
- Retroplacental
- Subamniotic
- Subchorionic
- Marginal
- Intervillous
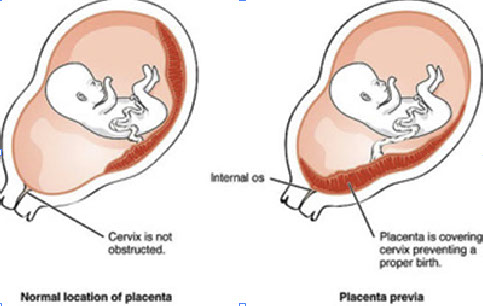
Placental implantation pathology
Placenta previa
- Placenta covers cervix and prevents a proper birth
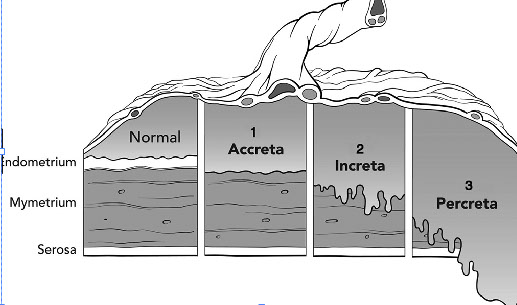
Placenta Accreta
-
Placenta Increta
-
Placenta Percreta
- goes through the serosa


1 - 4
<
>
Twins
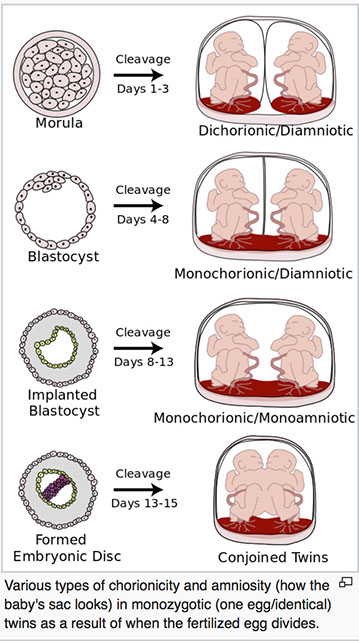

Comparison of zygote development in monozygotic and dizygotic twins. In the uterus, a majority of monozygotic twins (60–70%) share the same placenta but have separate amniotic sacs. In 18–30% of monozygotic twins each fetus has a separate placenta and a separate amniotic sac. A small number (1–2%) of monozygotic twins share the same placenta and amniotic sac. Fraternal twins each have their own placenta and own amniotic sac.
Intra-Uterine Growth Restriction (IUGR)
- may be 2/2 maternal hypoxia (heart dz, hemoglobinopathies, lung dz, smoking), reduced perfusion of intervillous space (HTN, vascular dz), villous exchange (confined placental mosaicism), fetal circulation (TTT, fetal cardiac failure), fetal metaolic problems
- in moms c HTN, see maternal vasculopathy, inc syncytial knots, infarcts
- in normotensive moms - see chronic vilitis, inc perivillous fibrin, maternal floor infarct
Listeria monocytogenes: facultative aerobe, Gram pos rods, motile, present in animal feces; worse earlier in preg
- MC is serotype 4b in humans
- 20x more common in preg, if infected kills up to 1/3, occur ost often in Hispanic women (from consuption of soft cheeses)
Dx: placental culture, gram stain of meconium, culture of neonate
Staging of Chorionitis:
1 - acute subchorionitis or chorionitis
2 - acute chorioamnionitis (inflam in fibrous chorion and/or amnion)
3 - Necrotizing chorioamnionitis (karyorrhexis of PMN, amniocyte necrosis and/or amnion BM hypereosinophilia)
Early Gestational Sac
Can see the trophoblastic shell around the outside edge, the stalk with the embryonic sac and the yolk sac
- is easily recognized when you see the embyo, but that is relatively uncommon
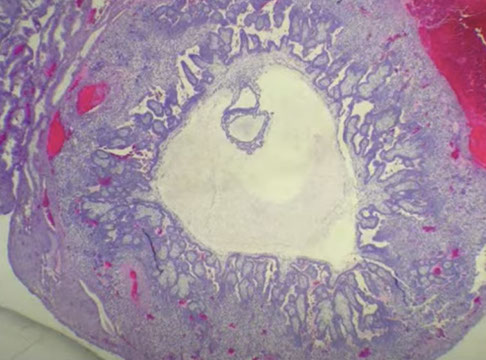
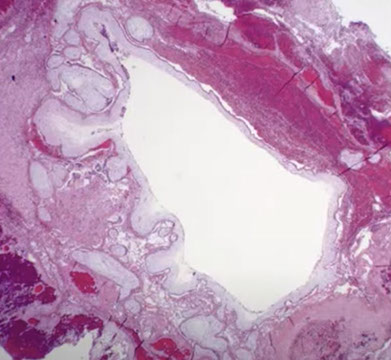
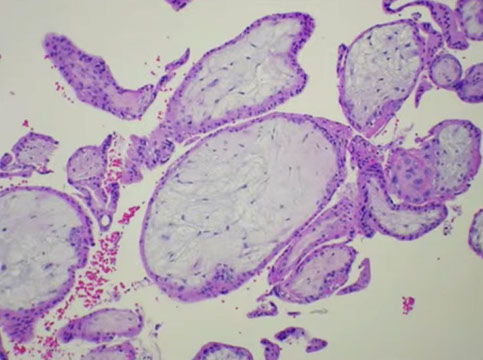

Early gestational sac, here can see the trophoblastic shell around the outside edge, the stalk with the embryonic sac and the yolk sac
Early gestational sac, usually never see more than a single cavitated villi
Early gestational sac, symmetric swelling with attenuation (no hyperplasia), without cavitation. Swelling like a balloon, causing thinning of the trophoblast layer. Still has strands of stroma
3 - 3
<
>
Necrotizing funisitis
.01% of deliveries, but up to 1/2 assoc c SB??
Grossly has barber pole appearance
Micro: stripes from necrotic inflm debris in arch around vessels
Hematogenous infx
transplaental from mamas blood, usually viruses and causes villitis
Syphilis
large bulky edematous placenta, hypecellular changes c large immature villi, prolif vasc changes, acute or chorionic villitis, nerotizing unisitis, Must visualize to document to CDC
CMV
lymphoplasmacytic illitis, necrotizing vasculitis, hemosiderin, inclusions
Parvo B19
causes nonimmune hydrops, ha characterstic cherry red inclusions in nucleated rbc, edematous villi, minimal inflam, anemia, myocaritis, death, newborn hepatitis
Call physician c lots of candida
Villitis of Unknown Etiology (VUE)
up to 1/10, assoc c IUGR, IUFD, dicordant dichorionic twins, recurrence risk 17%, 60% reproductiv loss rate, histo-prolif lymphocytic villitis c destruction necrosis and vilous atrophy
- is a dx of exclusion
Maternal Floor Infarct (MFI)
up to 1/2 stillborn, 1/2 assoc c IUFT, 1/2 preterm, up to 1/2 get recurrece
Intervillous thrombi
composed of fetal and maternal blod
- if massive fetus can bleed out and mom can get abs to fetal ags that can affect next preg
Chorangiosis of Chorionic Villi
may be 2/2 minor placental hypoxia
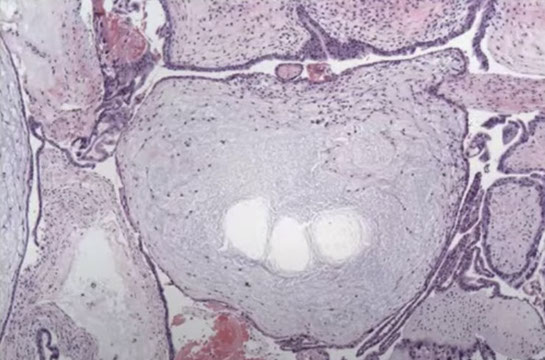
Chorangioma
most common type of placental neoplasm, with an
overall incidence of <1%. Typically, they are asymptomatic and are discovered incidentally at birth through gross examination of the placenta revealing a wellcircumscribed, spongy, red-brown nodule within the placental parenchyma, commonly at the periphery of the disc or directly below the cord insertion. Occasionally, multiple chorangiomas can be present. Most are discovered in mature placentas at 32-37 weeks’ gestation in mothers over the age of 30. Increasing maternal age has been correlated with increased incidence of chorangiomas. They are thought to develop as a reactive proliferation to hypoxia resulting in increased vascular endothelial growth factor production and angiogenesis. While most are incidentally found and have no clinical consequences, large chorangiomas (>4 cm)
may be associated with intrauterine growth restriction (IUGR), arterio-venous shunting, and fetal cardiac failure. Chorangiomas express GLUT1, similarly to
infantile hemangiomas, and may occur concurrently. While debated in the literature, some studies have shown a possible association between infantile hemangiomas and chorangiomas and even speculate that infantile hemangiomas may result from endothelial cell shedding from chorangiomas or hormonal influence during development.
Micro: well-circumscribed vascular proliferation composed of numerous capillaries. Examination of the nodule under high power reveals that the capillaries are surrounded by pericytes with no cytologic atypia. Mitotic figures are not readily identifiable. Surrounding the nodule, there are benignappearing cytotrophoblasts and syncytiotrophoblasts with appropriate syncytial knotting for gestational age. The few villi present surrounding the nodule are small,
mature, and appropriately developed for a third trimester placenta with a normal amount of vessels present
Chorangioma

Decidual vasculopathy
Normally, arteries transform into big channels through trophoblasts that invade and destroy arterial walls, making the vessel unable to vasoconstrict (muscular layer destroyed later)
- called lack of physiologic constriction if this doesn't happen
- also see atherosis, fibrinoid necrosis and vasculitis
- assoc c Gestational hypertension (as in pre-eclampsia or HELLP syndrome)
Decidual vasculopathy


Maternal Vascular Malperfusion (MVM)
- aka placental insufficiency, maternal vascular underperfusion, placental ischemia, Tenney-Parker changes
Abnormal perfusion of the placenta due to maternal vascular pathology
- not just underperfusion - high velocity / turbulent blood flow too
Prevalence: 8% in healthy nulliparous women
- 25-50% of pregnancies complicated by HTN
- 25% of pregnancies of SGA
- 50% of all preterm births (indicated and spontaneous)
Risk for complications
- 4.5x higher risk of developing preeclampsia or delivering a SGA neonate
- 50% have adverse pregnancy outcomes: SGA, PTB
Clinical association:
- HTN (preeclampsia, gestational, and chronic HTN)
- DM
- SLE
- APL syndrome
- oligohydrammnios
- abnormal Dopplers
- Decreased PAPPA and PIGF
- HIV
Fetal / Infant clinical associations
- Fetal growth restriction (1/4 of all IUGR fetuses are 2/2 MVM)
- Preterm birth (indicated and spontaenous, estimated to be in 50% of PTB)
- Neurocompromise (CP, IVH, hemorrhagic infarcts)
- Bronchopulmonary dysplasia
- Fetal mortality (main contributor of IUFD in up to 35%
Gross:
- small placenta by weight and dimensions (<10th percentile)
- thin umbilical cord (diameter <0.8 cm at term(
- placental masses consistent with infacts
- infarction hematomas / rounded intraplacental hematomas
- increased fibrin / fibrinoid
- acute, subacute, or chronic abruption
Micro
- Decidual arteriopathy (fibrinoid necrosis, acute atherosis, incomplete or transformed basal plate arterioles, thrombi in decidual vessels)
- decidual laminar necrosis
- chorionic microcysts / pseudocysts
- accelerated villous maturation
- increased syncytial trophoblastic knots
- distal villous hyperplasia
- placental infarcts (usual and / or hypertensive types; infarction hematoma / rounded intraplacental hematoma)
- increased perivillous fibrin / fibrinoid
- features of acute, subacute, or chronic abruption
Grading of MVM
Grade 1 (mile to moderate)
- normal placental weight, <30% involvement by pathology, no more than 1 marginal infarct)
Grade 2 (severe)
- placental weight <10th %tile
- >30% involved by pathology
- multiple or large central infarcts
Fetal Vascular Malperfusion (FVM)
- in ~6% of placentas, increases in prevalence with adverse perinatal outcomes - up to 16%
- several histologic pattern seen, such as thrombosis, avascular villi, villous stromal-vascular karyorrhexis, intramural fibrin thrombi, stem villous vascular obliteration
- may be caused by abnormal cord insertion, length, and coiling
-- can be assoc c maternal vascular malperfusion, such as preeclampsia, hypercoagulable states, lupus anticoagulant, and sometimes diabetes
- fetal cardiac dysfunction/malformations and severe fetal inflam response in setting of ascending intrauterine infx have been attributed [1], as well as low apgar scores, fetal growth restriction, hypotonia, hyporeflexia, preterm birth, adverse neurologic sequelae (intracranial bleeding, stroke, CP)
- increased perinatal mortality, esp stillbirth
- increased prevaluence of congenital abnormalities
**Caveat: prior intrauterine fetal death with degenerative changes post demise in the placenta results in a similar picture
Sx: may cause IUGR, poor perinatal outcome, fetal demise, and neurodevelopmental sequelae
Gross: light or heavy placenta
- marginal or velamentous insertion of the cord
- other cord abnormalities, long cord >80 cm or <35 cm, or >3 coils per 10 cm with deep grooves in Wharton jelly, or thin cords (more prone to compression), or true knots, hypercoiling, visible thrombi
- avascular villi may appear as pale triangular foci, esp after fixation
Micro: multiple patterns may be seen, such as:
- thrombosis of the fetal chorionic plate or umbilical cord vessels (can be seen grossly)
- mural organizing thrombosis with lamination with vascular muscular degeneration and extasia
- calcifications in older thrombi, or complete luminal obliteration in remote thrombotic lesions
- stem vessel obliteration (fibromuscular sclerosis) with thickening of vascular walls with luminal obliteration, usually close to the cord insertion site
- intramural fibrin deposits (usually non-occlusive)
Avascular villi seen with prolonged occlusion
- terminal villi lose villous capillaries and get stromal fibrosis
- syncytiotrophoblasts preserved as intervillous maternal circulation is maintained
-- now classified as small foci if at least 3+ foci of 2-4 terminal villi with total capillary loss, and as large foci if >10 villi affected
Villous stromal-vascular karyorrhexis (aka hemorrhagic endovasculitis) also seen with villous endothelial karyorrhexis with congestion, RBC extravasation, stromal apoptosis, separation of endothelial lumens
- must be seen in 3+ foci of 2-4 terminal villi with karyorrhaxis of fetal cells
FVM grtoss

FVM

Figure 5. Stem villous vascular luminal obliteration with markedly thickened muscular wall; fibromuscular sclerosis
Figure 6. Nonocclusive intramural fibrin with developing thrombosis [1]
http://www.archivesofpathology.org/doi/pdf/10.5858/arpa.2017-0212-RA

Figure 9. Villous stromal-vascular karyorrhexis ; Figure 10. Villous stromal-vascular karyorrhexis. Loss of integrity of the vascular wall; fragmentation and extravasation of red blood cells in the stroma with early septation [1]
FVM
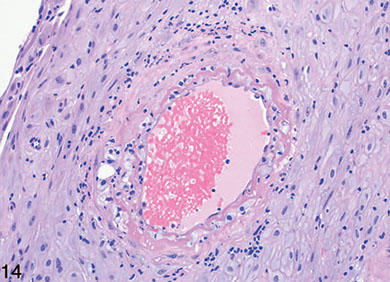
Figure 14. Decidual acute atherosis showing fibrinoid necrosis, lipidladen histiocytes, and perivascular lymphocytes [1]
FVM
Vacuolated amnionic epithelial (change) cytoplasm
Assoc c gastroschisis and omphalocele
- amnionic epithelial cells have fine uniform extensive vacuolization
- doent affect placental func
- vacuoles are lipid-laden, but dont no y, vacuoles only seen later in gestation (after 20 wks)
Vacuolated amnionic epithelial (change) cytoplasm

Trophoblastic Tumors
There is a differentiation pathway, similar to hemepath
Previllous trophoblast --> villous cytotrophoblasts
Villous cytotrophoblasts to syncytiotrophoblats, transitional extravillous trophoblasts, and trophoblast column
All trophoblasts are GATA3 positive
Risk factors: Maternal age <20 (RR 1.5), or >40 (RR 5.2)
- Race: esp in Indonesia, Japan (Asian women in US still have higher risk)
- Prior molar pregnancy
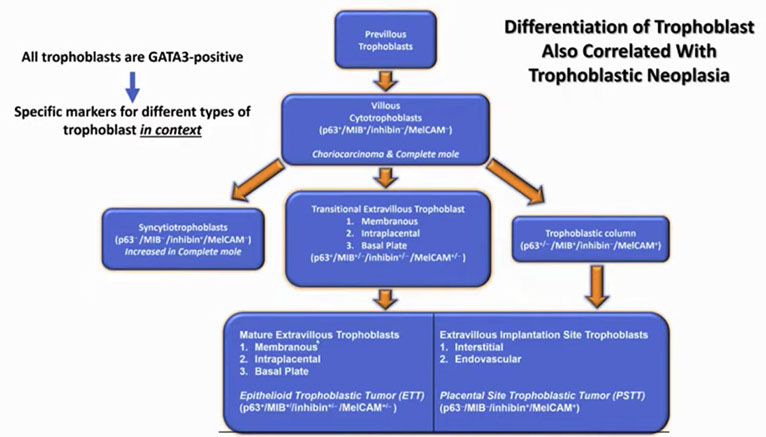
Trophoblast differentiation
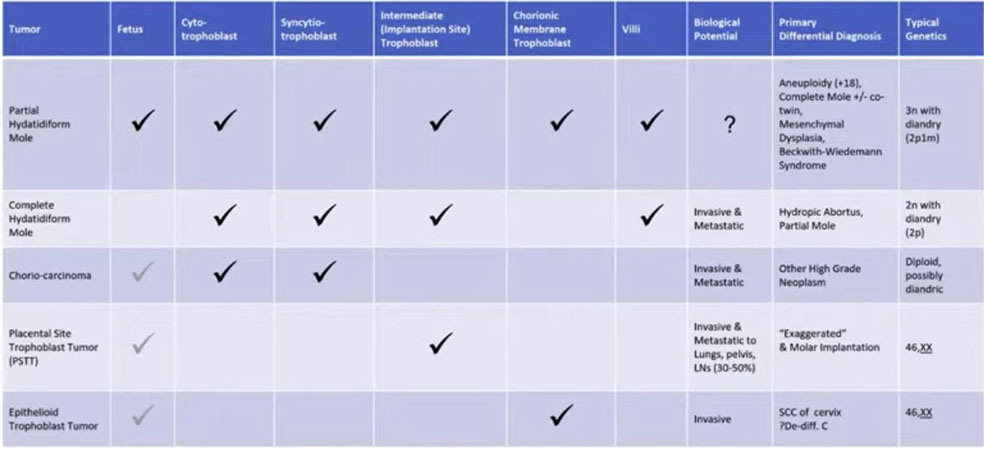
Nosology of trophoblastic tumors
1 - 2
<
>
Exaggerated Placental Site
IHC: (+) hPL (strong pos), Mel-CAM (strong pos), hCG, HLA-G, inhibin, CK18, CD10
- neg: p63, low Ki67 (<1%)
DDx: PSTT )presence of villi or fetal parts; absence of proliferation; less cytological atypia; abundant multinucleated IT; b-hCG continues to decline)
Exaggerated Placental Site


Placental Site Nodule (PSN)
Lesions of chorionic-type intermediate trophoblasts (IT), usually small
- 40-50% of PSNs are found in cervix; Always incidental findings
IHC: (+) p63 (very strong pos), hCG, HLA-G, Mel-CAM, inhibin, CK18, CD10
- neg: Cyclin E, hPL (var), Mel-CAM (var),
- low Ki67 (<5-8%)
Tx: Complete excision after surgical procedures
No clinical follow-up required
DDx: ETT

PSN`
Image A: Well-circumscribed lesion with acellular material in the center
Image B: Hyaline material containing scattered chorionic-type IT
Image C: Chorionic-type IT with smudged nuclei,indistinct cell border, mitosis is absent
Atypical Pleacental Site Nodule
More cellular atypia than regular PSN
- may be connective lesion bwt PSN and ETT
Placental Site Trophoblastic Tumor (PSTT)
Rare, caused by transformation of intermediate trophoblast at implantation site to cancer, usually after a long normal term pregnancy in pts of reproductive age
- presents as amenorrhea or abnormal bleeding with slightly inc b-hCG that can produce virilization
- tend to involve LUS and can mimic primary cervical ca
Gross: poorly defined soft mass in myometrium that can perforate uterus
Micro: Medium-sized spindly to polygonal cells c irreg borders (intermed trophoblasts) invading myometrium in nests, can have intranuclear pseudoinclusions, rare syncytiotrophoblasts can be seen
- similar to what is seen in normal pregnancy, vessel walls are infiltrated by intermediate trophoblasts, and deposit fibrinoid material in vessel wall and interstitium
- may be surrounded by lymphs
- no cytotrophoblasts or villi
- lots of fibrinoid stuff deposited and invasion of BV's
- infiltrative margins, low mits
IHC: (+) hCG (multinucleated cells), HLA-G, hPL, keratin, Mel-CAM, inhibin, CK5/6 (focal)
- negative PLAP, p63 (low)
- Ki67 >10%
Genes: diploid
Tx: curretage / hysterectomy, monitor b-hCG,
- 1/10 die from mets to lung, liver, brain
Px: worse c >5 mits/hpf, high Ki67 (>50%), inc celluarity, lots of necrosis, lots of cells c clear cytoplasma dn irreg nuclei, mets

Placental Site Trophoblastic Tumor. The image shows intermediate trophoblastic cells with pleomorphic nuclei deeply penetrating the myometrium. A multinucleated giant cell is also present.

PSTT
Epithelioid Trophoblastic Tumor (ETT)
Arises from chorionic-type intermediate trophoblastic cells
- b-hCG should not be as elevated as in chorioca ((?? per Webpath.com, levels of hCG are elevated ??)
Micro: atypical cells, tends to have a pushing pattern (vs PSTT which is infiltrative), very cellular and geographic necrosis,
IHC: (+) p63 (very strong pos), inhibin, CK18, Cyclin E (strong pos), hCG, HLA-G
- neg: hPL (var), Mel-CAM (var)
- low Ki67 (>10%, <25%)
DDx: may be mistaken for squamous cell carcinoma
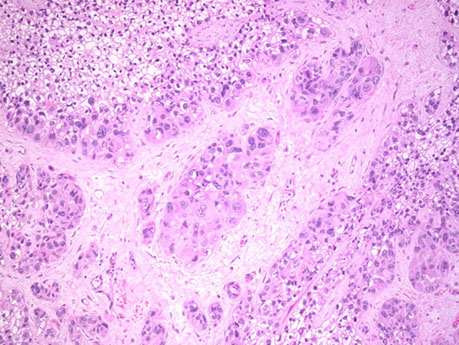
Epithelioid trophoblastic tumor. Tumor made of intermediate trophoblastic cells in nests and sheets in a hyaline matrix

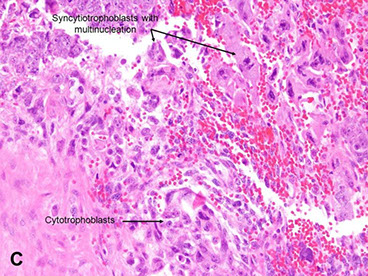

Choriocarcinoma
Comes from trophoblastic cells after pregnancy (either normal or abnormal)
- 1/2 assoc c moles (esp complete moles [1/40 chance])
- 1/4 from spontaneous abortions
- 1/4 from normal pregs (1/100,000)
- smalll % from ectopic pregs or teratomas (~2%)
- risk factor: being Nigerian, type A blood
- present c bleeding, foul-smelling discharge, very inc hCG (~18k) - which causes rxns in other organs (Arias-Stella rxn, breast duct hyperplasia, etc etc)
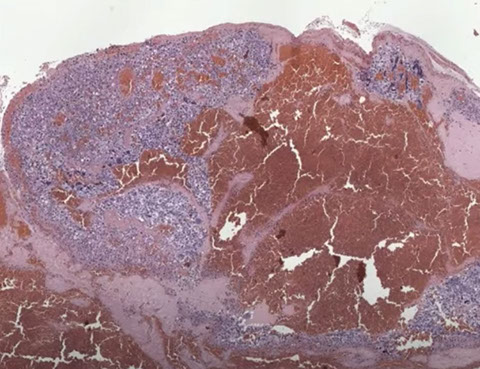
Gross: soft, fleshy hemorrhagic and necrotic, can be big or small
- sample the blood clot, especially at the periphery
Micro: Biphasic (all are cytokeratin and GATA3 +)
- cytotrophoblasts (CT, small, round mononucleated cells c clear cytoplasm) and intermediate trophoblasts (rIT, edder cytoplasm, has morphology of other 2 types??)
- syncytiotrophoblasts (ST, multinucleated) c variable atypia, pleomorphism, hyperchromasia, big nucleoli
- lots o mits, invades down to sm muscle and into vessels
- since doesn't make vessels, see lots of necrosis with only viable tumor found around periphery
- no villi are present
IHC: (+) CK, CEA
- int trophoblasts: (+) HLA-G, hPL, Mel-CAM (CD146)
- cytotrophoblasts: (+) p63
- syncytiotrophoblasts: (+) hCG, weak aPL
- mononucleate cells have high Ki67 (>90%)
- negative PLAP
Genes: Diploid (and diandric in CHM)
Tx: Chemo very successful
- monitor hCG
DDx: previllous trophoblasts in early gestations
- Persistent trophoblastic disease following a complete mole (no villi due to sampling, residual molar implantation site, choice of terminology does not have clinical impact)
- Placental Site Trophoblastic Tumor (PSTT) or Epithelioid Trophoblastic Tumor (ETT) ["syncytiotrophoblast-poor" choriocarcinoma]
Px: Will survive if given chemo and tumor just in uterus; drops to 8/10 survival if pt has mets
- Aggressive, rapidly invades and mets (to lung, vag, brain, liver, kidney, bowel; rarely mets to embryo (kiddo)
- worse if >39 yo mom, higher hCG, blood group B, larger size, mets
- better if lots of inflam bwt tumor and stroma
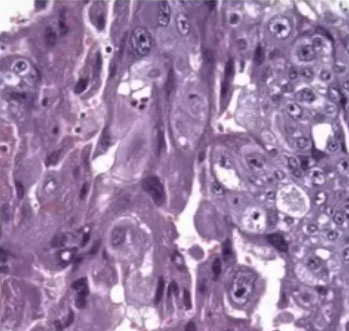
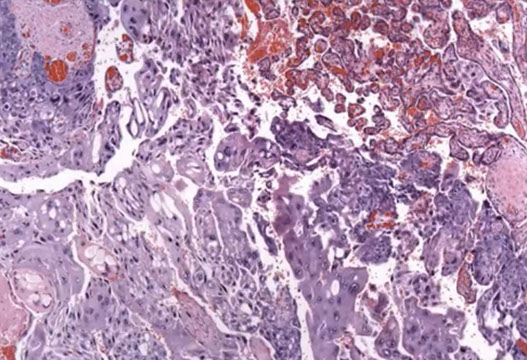

Choriocarcinoma, cytologic features
Choriocarcinoma can arise from within a placenta kind of as an in-situ lesion that looks like a white nodule; this is an example of an intraplacental choriocarcinoma in term non-molar placenta
Intraplacental choriocarcinoma in term non-molar placenta. See here how it is associated with the normal placenta
Mimicker of choriocarcinoma: Early gestation with trophoblast shell in hematosalpinx / ectopic pregnancy. Can see the shell of the trophoblast with the hemorrhage
1 - 4
<
>
Persistent Gestational Trophoblastic Disease
Usually a clinical dx, must meet 1 of these criteria:
1) 4 consecutive values v inc hVG over 3 weeks, 2) >10% rise in hCG in 3 samples over 2 weeks, 3) persistently inc hCG 6 mo after evacuation of malor preg
- persistently inc hCG could be retained molar villi, invasive mole, or chorioca
- if only atypical trophoblastic cells and no villi on curettage, could be trophoblast of very early pregnancy, retained molar tissue, exaggerated implantation site, chorioca
Molar Pregnancy
Various degrees of hydropic changes, both can have grape-like villous clustering near end of molar preg
- classically have villous edema and concentric hyperplastic trophoblastic prolif
- when villous edema fully developed, central clearing or "cisterns" are seen
- molar pregnancies rarely fully developed nowadays due to US and early evacuation
- FISH and flow cytometry can help differentiate mole types (harder to differentiate complete mole from hydropic abortus on FISH) and PCR for short tandem repeats
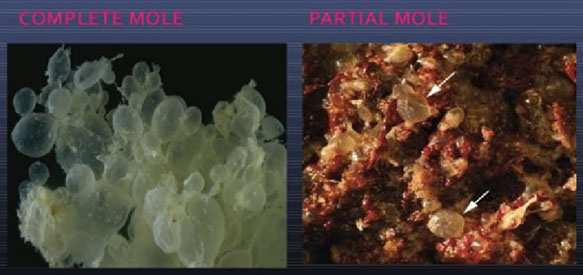



Gross molar pregnancy, complete (left) and partial (right)
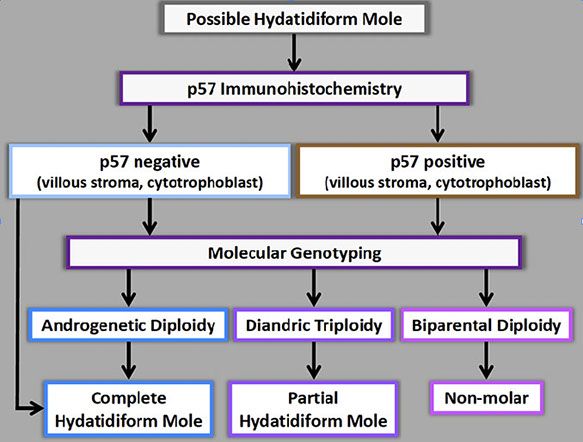
Summary of testing in molar pregnancies
Molar pregnancy evaluation algorithm
Non-molar specimen
Complete hydatidiform mole
Partial hydatidiform mole
1 - 6
<
>

CHM

Early complete CHM

CHM


Complete Hydatidiform Mole (CHM)
- grape-like clusters are only hydropic villi (no placental tissue)
*** Completely dad *** CD4 ***
Complete - Diploid, Diandric
Diploid, paternal origin only, no fetus
- uterine enlargement c markedly elevated B-hCG in 2nd trimester, possible hyperemesis gravidum, "snowstorm" on US
- risk factors: birth in 3rd world country, age >30 yrs, vit A deficiency, hx of prior mole
There is a Biparental CHM variant that are diploid but biparental
- Recurrent
- autosomal recessive
- maternal effect -- failure to establish maternal imprint
Gross: as the gestational age increases, the molar villi uniformly grow to macroscopic size, forming grape-like cysts. This entity is named because of the "water drop" cysts (Greek = 'hydatisia') cluster together forming a false conception (Latin = 'mole')
- no fetal parts present
Invasive complete moles, once known as "Chorioadenoma destruens" can be thought of as CHM with placent increta
- these are not readily detectable by curretage
- more freq in >40 yo and in underdeveloped countries
- hemorrhage may obscure villi grossly
- vascular space invasion common
- freq of persistent or metastatic GTD:
-- CHM >> invasive mole > chorioCA
- villi may be scarce
- pathobiologic mechanism unknown
Molar twins are also possible but rare [2]
- CHM of interest, but PHM possible as co-twin
- pitfall - iatrogenic admixture of CHM co-twin by curetage mimics PHM
- presence of normal co-twin often delays diagnosis
- higher pre-evacuation B-hCG; clinical symptoms likely
- poses obstetrical management issues, but successful delivery possible
Micro criteria are imperfect to differentiate molar and non-molar specimens c overlap in microscopic features and significant inter-observer variability
- clinicians expect report c molar vs non-molar, CHM vs PHM
Micro: Diffuse, circumferential trophoblastic prolif of uniformly enlarged, avascular edematous villi c marked prolif or trophoblasts that can be atypical
- early complete moles have uniform pop of villi that are big for gestational age, and villous edema c central cistern formation may not be present
- trophoblastic proliferation may also be focal or less developed in early dz
- villi have primitive appearance c inc mesenchymal cellularity and karyorrhexis and necrobiosis (not seen in partial mole or hydropic abortus)
IHC: (+) hCG (stronger than partial, less than choriocarcinoma), p53, CK, Inhibin, HLA-G
- negative p57 (loss of expression in villous stroma and villous cytotrophoblast, bc is a paternally imprinted gene transcribed from a maternal allele)
- p57 will be positive in intermediate trophoblast cells!
Genes: Androgenetic diploidy (~85% monospermic / homozygous); c 2 paternal and 0 maternal chromosome complements
- 1/2 diploid (46XX or XY, both coming from papa), little less than 1/2 tetraploid
- identity testing on FFPE using PCR on polymorphic STR loci on multiple cr's and amelogenin locus (XY)
- can also compare DNA patterns of villous and decidual tissue components (allele numbers and ratios: infer ploidy; allele patterns: determine maternal vs paternal source of cr complements)
Microarrays use SNP analysis to detect genome-wide LOH (uniparental) if derived from single sperm
DDx:
- very early gestational sac
- early abortus
- hydropic abortus
- partial mole
- twin gestation wiht complete mole as co-twin
Tx: cut it out, follow B-hCG to make sure that you got it all, CXR; contraception for pt
- if B-hCG level rises or plateaus, methotrexate tx added
Px: 1/5 are invasive or persistent (GTD), 1/20 get choriocarcinoma, 1/10 get mets to lung that cause respiratory distress
- better if some fibrin is bwt mole and uterus
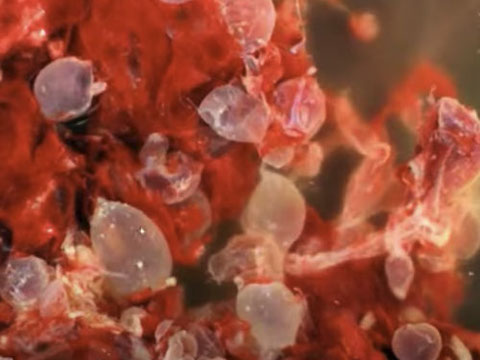

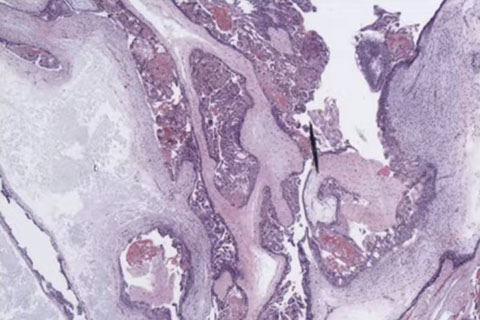


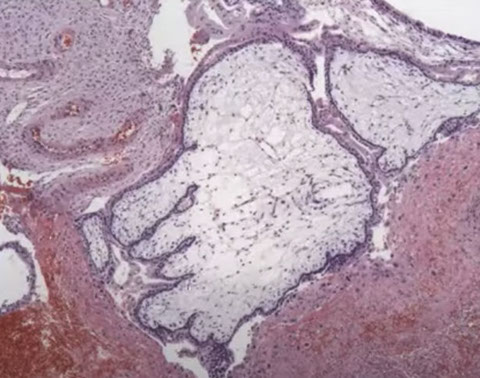
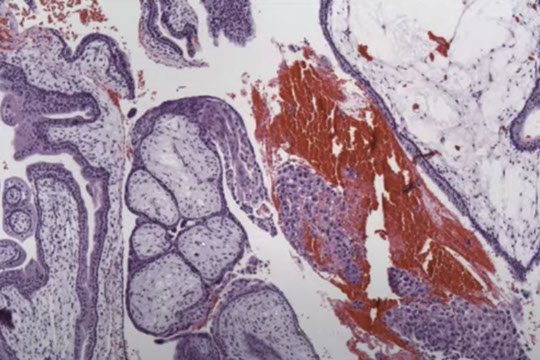


CHM gross
Histologic features of a CHM
Characteristic findings in CHM with cavitation and absence of fetal vessels. Note the contents completely degenerating and filled with protein rich fluid on the left.
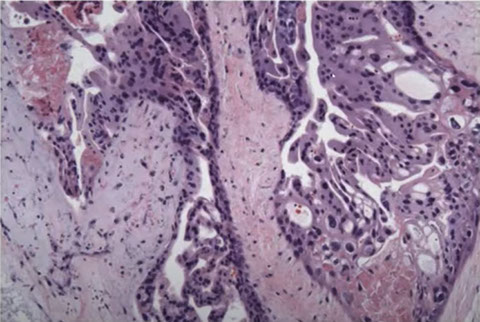
CHM. Trophoblast hyperplasia which is exuberant and circumferential
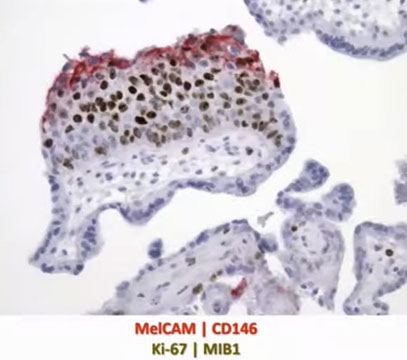
CHMs are distinct from the normal progression from proliferation to differentiation. At the tip it leaves the cells cycle and expresses CD146, which is expressed in the smooth muscle in the vessel wall of myometrium
CHM atypia can range from modest to severe
Molar implantation site. Distinctive - easy to see; more atypical than usual. May be your first clue. Pitfall: mimics choriocarcinoma or PSTT
Degenerating CHM have less atypia and hyperplasia. May be confused with non-molar hydropic villi.
Early CHM. Looks like toes on a foot with deep clefting. Note the blue myxoid stroma. Pitfall: Can be found in early gestations.
CHM. Has features of early CHM (like the blue myxoid stroma with the toes on the left middle) and of more classical CHM on the right
Early CHM. Seeing earlier and earlier CHMs due to ultrasound, here note the stromal and vascular degeneration. Pitfall is that you don't want to overcall missed abortion which can have similar degeneration.
11 - 11
<
>



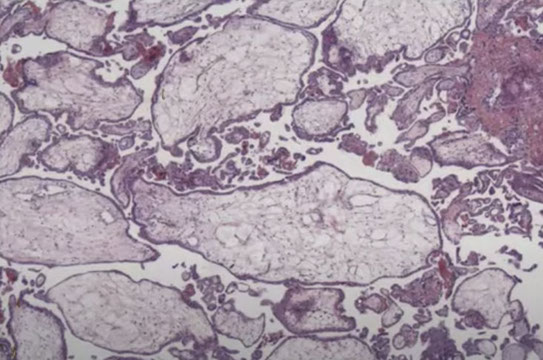
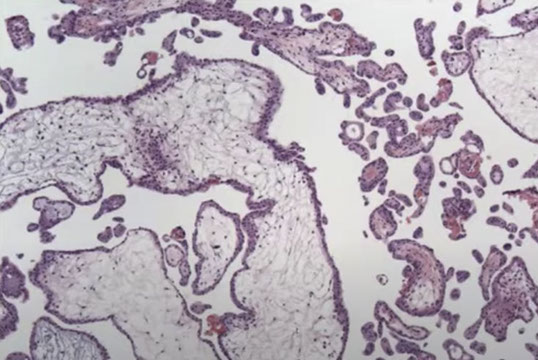
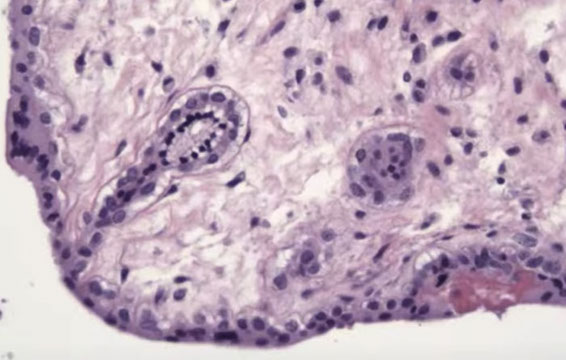
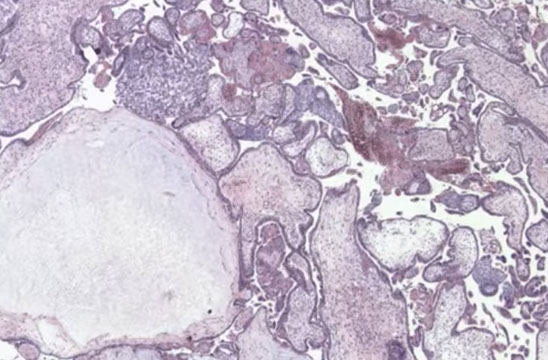
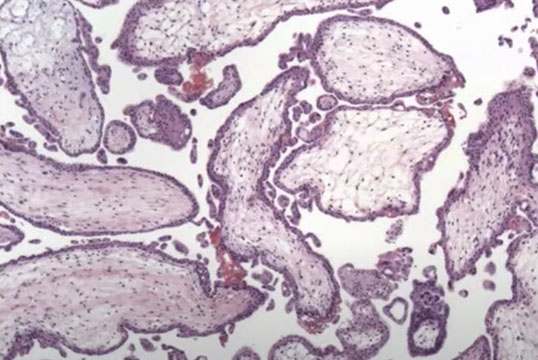


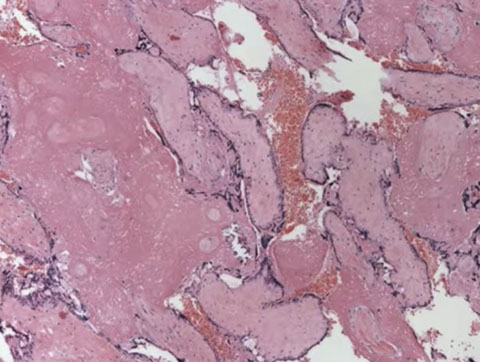



PHM gross. Hard to see the swollen cysts [2]
3-4 syndactyly is nearly always from a PHM
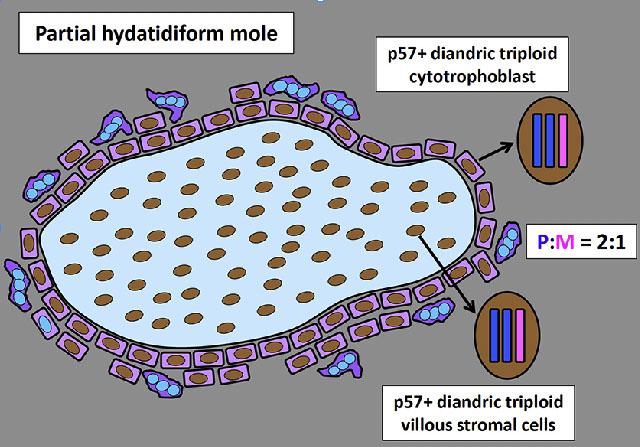
PHM. Intermixed biphasic population of villi by size. Proposed stringent threshold for villous enlargement >2.5 mm
PHMs have complex, irregular shapes in larger villi
PHMs have inclusions that reflect the shape complexity
PHMs with scattered cavitation of larger villi
PHM trophoblastic hyperplasia, proliferation commensurate to size and "tagging". Proliferation is just enough to keep up with the size of the villi
PHM fetal blood can commonly be seen, as well as an amnion and chorion (not pictured here)
Trisomy 13 is a frequent MIMICKER of PHM, as can be trisomy 21 and trisomy 11
Peristent PHM can look like funny retained villi
1 - 10
<
>
Invasive Complete Hydatidiform Mole
Molar villi invade into myometrium

invasive CHM (iCHM)
Partial Hydatidiform Mole (PHM)
- has normal placental tissue intermixed in grape-like villi and can see fetal parts
*** Mom and dad *** PT6 ***
Partial Tri-/Tetraploid
1/5 of all moles, low risk of choriocarcinoma, smaller than complete moles
- uterus can be small for date w/o enlarged uterus
- may accidentally be dx's as missed abortion by both clinician and pathologist
Gross: mix of vesicular and normal villi
Micro: mix of edematous and normal villi
- villous scalloping (irregular shape with trophoblast villi goes into villous stroma)
- do not develop uniformly hydropic villi, giving it a biphasic population of hydropic villi mixed c smaller fibrotic immature villi
- have stromal trophoblastic pseudoinclusions
- circumferential trophoblast proliferation less pronounced than in complete moles
- early partial mole suspected if some villi are dilated and not others, and borders usually more scalloped than late-term partial moles
IHC: (+) p57Kip2 (has maternal allele) in villous stromal cells and cytotrophoblasts, p53, hCG, CK, Inhibin, HLA-G
Genes: Diandric triploidy (~99% dispermic / heterozygous c 2 paternal and 1 maternal cr complements)
- tri- (>1/2 are XXY, <1/2 are XXX, few XYY) or tetraploid; 2 or 3 gene sets come from papi, 1 from mami
- some have trisomy 16
- good to get molecular to lock in dx
Tx: get CXR, monitor hCG
Px: <1/10 get gestational trophoblastic dz (GTD)
- low risk of choriocarcinoma or invasion
PHM


Hydropic Abortus
Presents as missed abortion c declining hCG levels
- grossly tissue scant, fetal parts usually not visible
- villi are uniform, round and occasionally fibrotic
- stromal vessels are rare and nonbranching, can have nRBCs
- trophoblastic hyperplasia is rare, but when present is polar
IHC: (+) p57 villous stromal cells and cytotrophoblasts
Genes: are diploid or aneuploid, occasionally triploid
Px: no inc risk of persistent dz

A and B, Hydropic nonmolar abortions show nuclear p57 staining in villous cytotrophoblast and stromal cells. C, Early complete hydatidiform mole with hypercellular, myxoid stroma, and circumferential trophoblastic proliferation. P57 immunostaining is absent in villous cytotrophoblast and stromal cells (D). Note the internal positive control in decidua in the upper portion of image (hematoxylin-eosin, original magnification x10 [A and C]; p57, original magnification x10 [B and D]).
Hydropic abortus


Early abortus (EA) c trophoblastic hyperplasia
asdf
EA with trophoblastic hyperplasia

Abnormal villous morphology (AVM)
asdf
Non-Molar AVM

Androgenic / biparental mosaic conceptions (MOS), some with CHM
asdf
MOS, +/- AVM

Metastasis to uterus
Rare, usually seen c widely disseminated dz and can cause sx
- MC mets come from breast (esp lobular carcinoma), colorectum and stomach
- MCC of intragenital mets are from ovary and salpinx, MC are serous tumors, usually go to serosa
- GATA3 picks up most breast ca (9/10), but not very specific (like mamaglobin)(GCDPF15 more specific but less sensitive)
- colorectal ca usually spreads by direct extension, but can also have mets through the blood
- most gastric ca that met are signet ring
References
1. Heider A. Fetal Vascular Malformation. Arch Pathol Lab Med 2017.
http://www.archivesofpathology.org/doi/pdf/10.5858/arpa.2017-0212-RA
2. YouTube. Dr. Quade. POC - molar pregnancies and gestational trophoblastic disease. https://www.youtube.com/watch?v=A39jmhPc3SI
3.
