Enzymes
Bone Chemistry
Cardiac Chemistry
Liver Chemistry
Pancreatic Chemistry
Bone Chemistry
ALP (Alkaline Phosphatase)
- also seen in liver; made by osteoblasts
- in pts c blood types B and O, inc serum ALP 2 hrs after fatty meal
- not sensitive or specific for bone dz
- may be falsely elevated in kids / elderly
- assay requires Mg2+ and Zn2+ for stability, which is inhibited by Ca2+, chelating agents, and inc [Zn2+]
- all types lose activity over 60 C, except for placental (seen from 20 WGA to ~ 1 wk post-partum) and germ cell variants
-- Regan variant is like placental, but assoc c neoplasms
- highest levels in bone variant seen c Paget's disease and Rickets (inc osteoblast activity)
Can measure diff in ALP activity in normal sample and one incubated at 56 C for bone fraction (heat sensitive)
- Neuraminidase removes sialic acid residues on protein and moves bone fraction
- Ficin breaks up enzyme bound to proteins
- Anti-placental Ab can differentiate placental from Regan fractions
ALP Assay- (requires 8 hr fast)
- lympholization and reconstitution, verapamil, estrogens, androgens, hepatotoxic / cholestatic drugs inc; a little bit of hemolysis doesn't affect; clofibrate, sulfas, malnutrition, azathioprine, estrogens / androgens dec
Colorimetric assay: p-NPP
Fluorometric R+D: 4-methyllumbelliferyl phosphate, FDP
Bowers and McComb kinetic (**the standard**): no bilirubin interference, forms bright yellow p-nitrophenylate product
May be able to separate bone from liver portions by incubation at 56 C
Cardiac Chemistry
Creatinine Kinase
Found in striated muscle of heart, brain and other tissues to a lesser extent
Additionally found in cytoplasm and mitochondria
-catalyzes formation of ATP and is required for contraction of muscle
Dimeric structure: M-muscle, B-brain
All isoenzymes weigh about the same (40,000 D)
In healthy people, the total CK measured is due to MM (almost exclusively) and is the result of physiological turnover of muscle
Macromolecular CK exists, but has no known clinical significance
Nomenclature based on subunits or electrophoretic mobility:
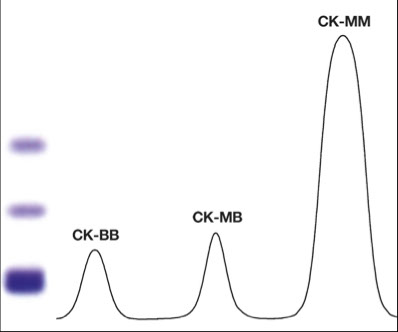
CK-MM: skeletal isoenzyme (CK3, slowest migrating)
CK-MB: cardiac isoenzyme
CK-BB: brain isoenzyme (and intestine)
- abnormal CK types: Macro CK (CK-Ig complex; seen in older women); mitochondrial CK
Soluble substances appear inversely proportional to size, such that small myoglobulin appears rapidly and larger LDH later
CK-MB has high specificity and sensitivity as a cardiac marker
- normal results of CK-MB cannot be used to r/o acute MI
- if CK-MB to total CK ratio >5% it is likely cardiac
Total Assay Methods of CK
Coupling reactions
Creatine + ATP --> Creatine Phosphate + ADP
- CK is unstable in serum and activity is lost due to SH group oxidation at the active site of the enzyme
Forward reaction (Cr --> CrP)
Reverse reaction (CrP --> Cr)
All commercial assays are based on this
Proceeds 6x faster than forward
Storage
Daylight inactivates CK (need to add Mg)
7 days at 4°C
30 days frozen
Must be free of hemolysis, serum or heparinized plasma
Physiologic variations
Reference: Oliver-Rosalki
Commercial methods vary in choice of buffer and source of G6PD
CK fractionation
No reference method
Electrophoresis best (alkaline pH, fluorescent stain)
Anode- BB-MB-MM- Cathodic point of application
Diasdvantage: long TAT, labor intensive, no adaptable to analyzers in emergencies and requires interpretation
Immunochemical methods
Measures catalytic activity
Immunoinhibition of Anti-M, assumes absence of CK-BB
Activity of CK-MB is doubled from that measured
Low sensitivity and specificity
Mass assays
Measure concentrations of CK-MB
Sandwich method
More sensitive
Advantage: unaffected by hemolysis, sample stability, fast TAT, automation
CK relative index
- Used to discriminate between physiologic variations in CK concentrations
[CK-MB]/[total CK](100)=%
- Physiological: <3%
- Equivocal: 3-5%
- Consistent with myocardial necrosis: >5%
Blood must be sampled 8-36 hours of symptom onset to be used
Myoglobin
Oxygen bearing heme protein found in skeletal muscles and heart
- No tissue isoenzymes
- Earliest marker of MI
- About 3 hours before CK
Nonspecific marker and is cleared by renal filtration
Negative test results are not helpful
- Doubling in 2-4 hours indicative of MI in absence of muscle trauma
Lactate Dehydrogenase
Catalyzes interconversion of lactic and pyruvic acids
Present 1500-5000x greater in tissue than serum
- LDH is a tetramer composed of 2 subunits, H (for heart) and M (for muscle)
-- LDH 1 is a tetramer of H subunits, while LDH 5 is a
tetramer of M subunits
- LD1 and LD2 predominate in heart, kidneys and RBC
-- in normal serum LD2 > LD1
-- in MI, hemolysis and renal infarct, called a "flipped pattern"[2]], bc LDH1 is more abundant in myocardium than LDH2, with ratio of ~45:40 (this ratio is also seen in RBCs, germ cells and renal cortex)
- LD 4 / 5 mean liver damage
Requires coenzyme NAD
Total enzyme is not specific to any particular disease
Isoenzyme fractions more diagnostic
Total LD Assays
Lactate + NAD <---> Pyruvate + NADH + H
Kinetic methods (older methods use end point dye)
Sources of error: Hemolysis, Storage (must be RT), Time limits
TnI and TnT
Vital role in diagnosis and risk stratification
- For diagnosis using troponins there must be cardiac ischemia present
- Not present in healthy individuals (though is only 99% specific, so 1 case in 100 will be treated for MI without actually having an MI)
Release kinetics mirror CK-MB, but more sensitive
- Draw immediately, then every 6-9 hours
Stay elevated in blood 5-14 days after MI
Very low values in patients without cardiac disease permit lower discriminator values for MI
TnI
Not elevated in patients with extreme skeletal muscle injury (marathon racing, Duchenne’s and chronic renal failure requiring dialysis)
- CK-MB and total CK are elevated
- Lower molecular weight than TnT
TnT
Not an early marker of MI
No major clinical difference, differences in assayed levels are due to calibration and assay differences
Troponin assay interference
Heterophile antibodies
- Endogenous antibodies directed against the proteins of nonhuman species
- Natural antibodies and autoantibodies that are polyreactive against heterogensous, poorly defined antigens of different chemical composition
- Low affinity or weak binding
- No clearly defined immunogen, antibody reacts with immunoglobulin from two ore more species or has RF activity
- RF-Rheumatoid Factor (false positives)
HAAA- Human Anti Animal Antibodies
- High affinity, specific polyclonal antibodies produced against a specific animal immunogen whole immunoglobulin of IgG or IgM class
- Strong binding of an antigen with a single chemical composition
- Produced in high titers
- Cross react with reagent antibodies by competing with the test antigen to produce a false signal
Autoantibodies
Only Roche tests for TnT due to patents
B-type natriuretic peptide (BNP)
Assayed as pro-BNP, the 108aa precurser, or BNP, a 32aa peptide hormone
- atrial cells make A-type natriuretic peptide (ANP) while ventricular cells make B-type natriuretic peptide (BNP)
Regulates blood pressure and fluid balance along with ANP
Heart releases BNP in response to ventricular volume expansion and pressure overload
Theorized a substantial release from failing heart into plasma (chronic marker)
BNP assays
BNP, pro-BNP and NT-pro-BNP are all measured by dual-site sandwich immunoassays
- Roche patented NT-pro-BNP
- Documented clinical equivalency with NT-pro-- BNP and BNP for patients with heart failure
- No FDA approved pro-BNP assays
No reference methods
Dual-site improves specificity and removes cross-reactivity from other structurally related peptides
RIA used in research, not practical in clinical setting
Specimen: EDTA; ideally tested within 4 hours







Liver Chemistry
Phase I: oxidation / hydroxylation
- cytochrome p450
Phase II: conjugation
Hepatic Urea Cycle
- metabolism of amine remnants of protein metabolism
- if defective, see dec BUN and inc ammonia
Heme Metabolism
- makes ~0.25 g per diem
Heme --> Biliverdin --> bilirubin (in blood) --> in liver, UDP-glucoronyl transferase 1 attaches glucuronic acid (glucuronidation)
- rate-limiting step is active transport into canaliculi
- in gut, reduced to urobilinogen (colorless), then oxidized to stercobilin (makes poo brown)
-- some urobilinogen gets returned to liver by portal vein, then gets excreted into bile or filtered into urine
--- inc urine urobilinogen: 1) inc bilirubin prod; 2) inc portal vein transport; 3) dec liver clearance
-- measuring urobilinogen not helpful in pts c known / suspected liver dz
Normal there should be no conjugated bilirubin in blood; if conjugated bilirubin reaches blood, undergoes reaction c lysine residues on albumin, forming a permanent bond bwt bilirubin and albumin called biliprotein or d-bilirubin.
Monoconjugated bilirubins (d-bilirubin and biliproteins) have much longer half-life than regular albumin (1 vs 20 days)
- thus can tell for a while if there has been impaired excretion of conjugated bilirubin (although conjugated bilirubin itself is cleared quickly from blood in ~24 hrs)
Unconjugated bilirubin cannot be excreted by kidneys; therefore, all bilirubinuria means underlying conjugated hyperbilirubinemia (?)
Silver stool of Thompson = white poo caused by biliary obstruction
Majority of plasma proteins produced by liver, c exception of immunoglobulins
- dec plasma proteins levels not necessarily indicative of liver disorders
- things that can cause a dec protein synth: AA availability, oncotic pressure, humoral factors
- regulates blood glucose through insulin / glucagon
- bile acids synth'd from cholesterol; levels may be altered c cholestasis
If conjugated bilirubinemia lasts for too long, binds to albumin and becomes d(elta)-bilirubin; that can persist for a while bc cant be extreted by kidneys or liver
- see jaundince in 79% Hep A, 30% Heps B + C (less common in kids)
- bilirubin > 15 means severe liver injury and poor px
Lab Methods
1) Diazo-colorimetric methods: bilirubin reacts with a diazo compound, forming a colored dye
- if accelerant (EtOH) not added, you're measuring conjugated (direct) bilirubin
- unconjugated bili doesn't react c diazo directly
2) Direct spectrophotometry (bilirubinometer): measures bilirubin absorbance at 455 nm and oxyhemoglobin at 540 nm
- need to correct for Hgb by subtracting 575 nm peak
- only measures total bilirubin
Prothrombin Time (PT) can be a good marker for hepatic injury
- can differentiate from vit K deficiency by injecting vit K parentally and seeing if it corrects
- PT is the best indicator of px in acute hepatic injury; assoc c very poor px if PT > 4 secs
Ammonia
In body, made from gut and sk muscle
Think liver failure if elevated; can be inc c variceal bleeds (excess protein [hemoglobin] in the gut)
- though in kids can be due to metabolic problems
Can cause neuro damage at very high levels
Needs to be chilled during transport to prevent hemolysis
Smokers can't smoke for a couple hours before this test
Proteins made by liver:
- almost all plasma proteins (except immunoglobulins) are made by the liver; including transport proteins (albumin, transferrin, lipoproteins), acute phase
reactants released in response to IL-6 (antiproteases,
complement) and coagulation factors
- if liver stops producing proteins, their levels fall at the rate of their half life
Transthyretin (prealbumin)- transportation of retinol, thyroxine binding
- has T1/2 of 1-2 days
Albumin - plasma oncotic pressure, transportation of substances and reservoir for AA's
- T1/2 of 20 days
a1-antitrypsin - inhibits protease
orosomucoid - transports drugs and mediates immune response
Factor VII - T1/2 is 6-8 hours, and is thus a good measure of acute liver damage,
haptoglobin - binds free hemoglobin
a1-macroglobulin - another protease inhibitor
ceruloplasmin - contains majority of copper, oxidizes iron
transferrin - transports iron from bowels
Lipoprotein X - abnormal protein erroneously measured as LDL produced with cholestasis
Liver enzymes:
Aminotransferases (AST and ALT)
- differing t½ of AST (16-18 h) and ALT (42-48 h) explain why AST is typically higher early in liver injury , but lower than ALT by time of presentation of most hepatocyte problems
- Pyridoxal phosphate is a necessary co-factor, esp for ALT; pyridoxine deficiency (EtOH abuse, malnutrition) or pyridoxine binders (chronic renal failure) can underestimate of ALT activity unless pyridoxal phosphate is included in reagents.
AST (Aspartate Aminotransferase or SGOT)
Converts aspartate to oxaloacetate
T1/2 = ~17 hours (~ 1 day)
- highest conc in heart, then liver; not as specific for liver as ALT; 20% in cytoplasm, 80% in mitochondria
- serum / plasma sample can be interfered c ammonium-containing compounds and hemolysis, c slight age (higher in kids, reverses at age 20 years, though can reverse in old age) / sex changes in the ref range
Higher concentration in mitochondria
- since EtOH is a mitochondrial poison, see higher AST in EtOH toxicity
ALT (Alanine Aminotransferase or SGPT)
Converts alanine to pyruvate
- ALT higher in cytoplasm than in mitochondria, though AST higher than ALT in cytoplasm
T1/2 = ~45 hours (2 days); has up to 30% day-to-day variation
- highest conc in liver, then kidney
- serum (not urine) sample can be altered c hemolysis, slight variation of ref range depending on age / sex (M>F, blacks > whites)
-- heparin can triple their levels
-- levels are lower in renal failure (how???)
Ratio of AST:ALT = deRitis ratio
> 2 in 4/5 pts c toxic hepattis (if AST > 3k, 90% due to a toxin)
< 2 in viral hepatitis
... a digression ...
Two types of phosphatases: alkaline (at pH 9) and acid (pH optimum is 5)
Acid phosphatases: TRAP and prostatic acid phosphatase (detects prostate cancer)
- TRAP: used to be used to distinguish red cell acid phosphatase from other phosphatases (prostate, bone)
Alkaline Phosphatase (ALP)
Family of enzymes that hydrolyze monophosphate esters at alkaline pH (10)
- membrane-bound enzyme found on canalicular surface of the liver
- also seen in bone (inc c bone growth & dz), pregs, and bile duct obstruction
-- bone isoenzyme made by osteoblasts (bone formation) --> very inc in Paget's dz of bone
- those c blood type Lewis B or O can have falsely elevated levels from intestinal ALP
- needs zinc and magnesium as cofactors
If ALP elevated in middle aged woman, get anti-mitochondria abs
If ALP elevated but GGT and 5' nuclease normal, suspect bone origin (esp Paget's in older pts)
Be able to differentiate different kinds of ALP
90% bone isoenzyme is inactivated c heating (*** bone burns ***)
- Obstruction of bile duct drainage causes dissolution of these bonds causing a progressive rise in plasma ALP
- Indicator of obstruction, but not level of obstruction
- biliary ALP is most sensitive marker of hepatic mets
Not released due to hepatocyte injury
Membrane-bound ALP has faster electrophoretic mobility (fast liver, macrohepatic, alpha-1) than non-lipid bound form
GGT (Gammaglutanyl Transferase)
Membrane-bound enzyme tof the biliary epithelial cells hat participates in glutathione metabolism and AA movement in glomeruli and intestines
- inc serum levels in renal dz in HTN / DM, males (bc of high levels in prostates), biliary obstruction. EtOH use, anti-convulsants
-- highest conc in kidney, then pancreas
- serum / plasma sample can be altered by lipemia or hemolysis, and reference range depends on pt age / gender
Best test to confirm an elevated ALP is from the bile ducts
Also found at Canalicular Membrane (same as ALP)
5' NT (5' Nucleotidase)
Inc c bile duct obstruction; found in biliary epithelial cells
- stays normal in bone dz / drug damage (useful in monitoring cancer progression during chemo)
Also can be used to confirm that ALP is from the biliary tracts, though not used that much bc GGT is more specific
a-Fetoprotein (AFP)
major plasma protein in early fetal life (concentrations in mg/mL range),
- levels fall to ng/mL by 2-3 months, tho regenerating hepatocytes also produce AFP
- pts recovering from hepatitis or with an acute flare of chronic active hepatitis may have values in the 10-50 x normal range
- Very high levels (> 100 x normal) are virtually pathognomonic of a primary liver neoplasm, either hepatocellular carcinoma or hepatoblastoma (in children), or germ cell tumors; however, such marked elevations occur in a small percentage of liver tumors.
Less specific:
AcetylCholineEsterase (AChE)
Inc serum levels c pesticide exposure (dec after repeat injury)
- variant form assoc c difficulty to revive pts from anesthesia
- shows poor liver function faster than albumin or other proteins
- serum / heparinized plasma sample has to be separated fast; slight age / sex ref range differences







Canaliculis
Sinusoid

Pancreatic Chemistry
Amylase
Enzymes that degrade complex carbohydrate molecules into smaller components
Secreted to aid in the digestion of starch from the salivary glands and the pancreas
- see 6 bands in electrophoresis, first 3 are salivary, the slowest are pancreatic
Gradient of amylase between pancreas and the plasma is high (sensitive for pancreatic injury, but not specific)
Values must be 3-5x that of the reference range limit
Elevated within a few hours of pancreatic injury and remains elevated for 2-5 days
- amylase sensitivity in pancreatitis is up to 98%; although on 3/4 specificity (many other causes of inc amylase [DKA, salpingitis, bowel inflam])
-- 10% of pancreatitis with normal amylase can be seen if pancreatitis is caused by hypertriglyceridemia
- think complication such as a pseudocyst if serum amylase remains elevated
Assay conditions are rigidly controlled to meet the requirements of amylase:
- Optimum pH: 6.9-7.0
- Ca2+ and Cl- are required cofactors
Amylase / creatinine clearance:
Elevated ratio highly specific for acute pancreatitis
When compared to serum or urine amylase, it is relatively insensitive for acute pancreatitis
Distinguishes between macroamylasemia and acute pancreatitis
- Macroamylasemia: elevated serum amylase without symptoms
- Serum amylase is high, but urine amylase is low
- caused by Ig-amylase complexes
Amyloclastic technique
Measure rate of hydrolysis of starch by amylase
Less defined substrate led to less consistent measurements
Methods
Turbidimetric (kinetic or fixed time)
Utilizes ability of amylase to decrease the turbidity of a substrate suspension by enzymatic degradation of the substrate into smaller units
Change in turbidity is proportionate to amylase activity
Nephelometric (kinetic or fixed time)
Measure increase in light measurements over a short time
Change in light sattering is proportionate to amylase activity
Iodometric (fixed time)
Ability of iodine to form a vivid blue color after a reaction with starch
Greater the amylase activity, the lighter the blue solution will be
Saccharogenic techniques
Depend on the measurements of monosaccharides or disaccharides liberated by amylase’s reaction with substrate
Modified for automated kinetic analysis
Reliable but time consuming, have high sample blanks and variable substrates
Requires correction for patient’s glucose concentration
Chromolytic techniques
Liberation of dye coupled to polysaccharides after a fixed time
Widely used on automated analyzers
Fast, precise, consistent and easy to perform
Release of small, water soluble fragments in such a way that the color can be measured spectrophotometrically
Substrate hydrolyzed by amylase and coupled reaction of substrate fragments to another molecule.
Measure at 405nm
Lipase
Enzymes that hydrolyze glycerol esters of long-chain fatty acids
Acts only at the ester-water interface
Must be run as emulsions
Found in two forms L1 and L2 (absent in normal person, high in those with pancreatitis)
If >3x normal value, it is specific and sens for acute pancreatitis than amylase
Values above reference range can be diagnostic
Elevated within a few hours of pancreatic injury and remain elevated for up to 2 weeks (longer than amylase)
- triglyerides dont interfere c lipase levels
Lipase techniques
Titrimetric
Cherry-Crandall method used olive oil emulsion mix
Older method
Colorimetric
Massion and Seligson method quantitates the liberated fatty acids after 1h of incubation and uses a methyl red indicator
Immunologic
Quantitative enzyme immunoassays and semiquantitative latex-agglutinination
Coupled enzyme spectrophotometric
Based on measurement of free glycerol
Can be affected by certain drugs
Turbidimetric
Requires highly stable, reproducible substrate with an appropriate initial absorbance
Kinetic measurements made either turbidimetrically or nephelometrically
Use colipase to enhance lipase measurements


Ranson's criteria

References
1. Notes, the U
2. Jiadal, I et al. Clinical Utility of Lactate Dehydrogenase. AJCP Feb 2015; p158-159
