Cervix
Anatomy and histology
Grossing
Arias-Stella Reaction
Atrophy
Basal cell hyperplasia
Hyper- / para-keratosis
Endometriosis
Herpes Simplex Virus (HSV)- cervicitis
Metaplasia
Diffuse Laminar Endocervical Glandular Hyperplasia
Lobular Endocervical Glandular Hyperplasia
Microglandular hyperplasia
Radiation-induced atypia
Reactive / Reparative Squamous Atypia
Endocervical polyps
Endometrial hyperplasia
Endocervicosis
Epithelioid Trophoblastic Tumor
Mesonephric hyperplasia
Nabothian cyst
Placental site nodule
Tunnel clusters
Rhabdomyosarcoma
- Sarcoma botryoides
Squamous Intraepithelial Lesions (SIL)
Superficially Invasive Squamous Carcinoma
Squamous cell carcinoma
Adenosquamous carcinoma
Adenocarcinoma In Situ (AIS)
Superficially Invasive Adenocarcinoma
Invasive Adenocarcinoma
Basaloid Squamous Carcinoma
Lymphoepithelioma-Like Carcinoma
Neuroendocrine carcinoma
Papillary Squamous Cell Carcinoma
Spindle Cell (sarcomatoid) carcinoma
Transitional Cell Carcinoma
Verrucous Carcinoma
Cervix
Anatomy and Histology
Divided into portio vaginalis and portio supravaginalis
Ectocervix - lined by stratified squamous cells
- found on the outer portion of cervix up to the squamocolumnar junction of the external os
- divided in basal, parabasal, intermediate and superficial layers
-- basal layer: dense chromatin in picket-fence pattern which are mitotically inactive and are Ki-67 neg
-- parabasal cells are a little bigger than basal layer cells bc have more cytoplasm; their nuclei are less condensed, they are mitotically active, thus (+) Ki67
-- intermediate cells have lots of clear cytoplasm from glycogen accumulation (don't confuse c koilocytes!!), have more vesicular nuclei
- superficial cells also have lots of clear cytoplasm, but have smaller rounder nuclei
-- superficial cells predominate c lots of estrogen, while intermediate cells seen more after ovulation
- can see hair follicles or other glands in ectocerivix
External os is slit-shaped in parous women
Squamocolumnar junction - found at the external os in prepubertal women, moves a little outward at menarche, and then moves up, as squamocolumnar metaplasia, toward internal os during reproductive years, then goes back out again towards external os again nearing menopause
- may undergo squamous epithelialization where squames go under glands and push them up and out; or can undergo squamous metaplasia where endothelial reserve cells proliferate
-- immature squamous metaplasia may look dysplastic, bc the cells have inc NC, lack maturation, and are hyperchromatic, but they are also relatively uniform c smooth nuclear contours (a Ki67 may help to differentiate [<15% in basal / surface cells of squamous metaplasia])
- transitional metaplasia resembles urothelium and has hyperplastic epithelium w/o maturation, inc NC, uniform oval nuclei c lots of grooves, no nucleoli, and swirling architecture, is usually assic c atrophy and will be (+) CK13/17/18 but CK20 neg
Endocervix - simple columnar mucinous cells c small compact basal nuclei (which can move apical during menses); ciliation increases towards internal os
- begins at the external os and ends at the internal os
- bcl-2 and CK17 decreases towards internal os
- clefts and glands in endocervix also lined by columnar cells and can be mistaken for adenoca
- can have inc stromal vessels from endometrium in upper segments, similar to lower uterine segment (but not as closely packed)
- Mesonephric remnants seen in 1/3 of normal cervix, and are tubular structes lined by single layer of cuboidal cells c round bland nuclei and red stuff in lumen
- decidual rxn can look like SCC, but is not pleomorphic, no connection to surface epithelium, no mits and does not express keratins
Internal os - endocervical canal widens and epithelium changes to lower uterine segment
Grossing
Loop Electrocautery Excision Procedure (LEEP) or Large Loop Excision of the Transformation Zone (LLETZ)
1. Id endo and ectocervical margins
2. Ink ectocarvical margin and stroma with 1 color, the endocervical margin with another
3. If cylindrical, divide into halves and section longitudinally
4. If saucer-shaped, section radially
5. TE the specimen
Cone Biopsy
Similar as LEEP, orient c stitch at 12, inking the stroma and ectocervical margins 1 color and the endocervical another, then section radially as a cylindrical LEEP specimen, submitting in series around the clock in 3 hour intervals (12-3, 3-6,..9-12)
- TE specimen




Transitional metaplasia of the cervix


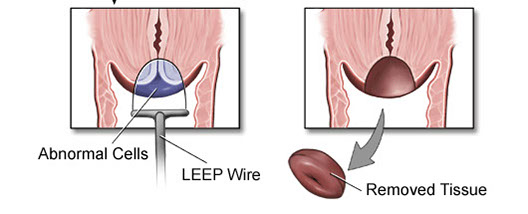
stroma ------
ectocervical margin
--------------endocervical margin
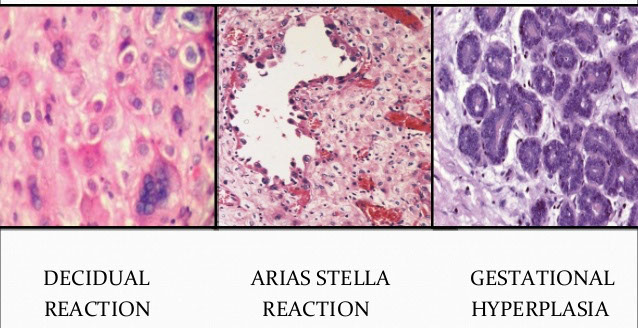
Arias-Stella Reaction
Found in up to 2/5 pregnancies, or in pts on OCPs or idiopathic
- affects superficial or deep glands and can be local or diffuse
- usually a focal finding, when seen in curetting is assoc c intrauterine and ectopic gestations
Micro: big cells that can look red or c big vacuoles or hobnailed, irreg nuclei sometimes hyperchromatic
- can form papillary tufts or cribriform
- low mits, rare pseudoinclusions in nucleus, occasional decidualization of stroma
- can mimic AIS or clear cell ca
Arias Stella Reaction

Atrophy
Altered squamous maturation 2/2 hormonal deficiency (menopause or prepuberty)
Micro: can produce several patterns:
Typical: thin epithelium c lack of maturation c uniform basal and parabasal cells and inc NC, dense chromatin, no / rare mits, little-mild pleomorphism
Atrophy with partial maturation: cytoplasmic glycogen and large evenly spaced nuclei and scattered mits
Pseudokoilocytosis (aka postmenopausal squamous atypia) hyperchromatic nuclei c even chromatin, binucleation, and cytoplasmic halos c more uniform size and shape than HPV
IHC: Ki67 <20% in basal 1/3, <5% in middle 1/3 and absent in superficial cells (vs in SIL)
- negative: p16 (vs SIL)

Cerix c atrophy
Basal cell hyperplasia
Assoc c OCPs, IUDs, preg, inflam, repair, see inc thickness of basal and parabasal layers c enlarged, vertical, oval nuclei w/o pleomorphism or hyperchromasia
- picket-fence arrangement normally seen in basal layer is lost, c normal overlying squames
Hyperkeratosis and Parakeratosis
Usually assoc c chronic irritation, appears as a focal / diffuse white epithelial thickening
- can (infrequently [1/20]) occur c SCC, seen in routine Paps in up to 1/10
Micro: acanthotic c elongated rete ridges, little glycogen, sometimes prominent granular layer
Endometriosis
Can be superficial, polypoid or deep
- superficial type assoc c prev cervical trauma (bx, curettage, abortion, vag delivery)
- polypoid type has exophytic growth pattern and is assoc c hormone therapy and soybean phytoestrogen
- stroma can have lots o inflam, bleeding, sm hyperplasia and fragmentation simulating AIS
IHC: (+) Bcl2 and PAX-2 (neg in AIS)
- p16 and MIB1 usually (+) [and therefore not very helpful]
Herpes Simplex Virus (HSV) Cervicitis
In early phases see homogenous cells in basal layer c nuclear or cytoplasmic vacuolization, which then moves up into intermediate cell layer and forms bullae that rupture and ulcerate causing epithelial cell necrosis and inc inflam
- ground glass chromatin in nuclei
Follicular cervicitis
Assoc c chlamydia infx
- see tingible body macrophages in lymph nodes
Follicular cervicitis c macrophage inclusion, assoc c chlamydia infx

Metaplasias
Tubal, endometroid and tuboendometroid metaplasias are idiopathic
- can mimic cancer, but metaplasia usually has more even chromatin, no hyperchromasia, low mits (ki67 <10%)
- metaplasia c scattered p16, and is Bcl2 strong + (negative in adenoca), and CEA not very helpful but more pos in AIS
Atypical Oxyphilic Metaplasia
Large polygonal or cuboidal cells c lots of red cytoplasm and large dark nuclei (sometimes multinucleation), big nucleoli, apical snouts, cytoplasmic vacuolization, rare mits
- cells c slightly irreg contour in monolayer with occasional tufting
Endometrioid metaplasia
Changing of endocervical epithelium to columnar cells, may be pseudostratified
- low mits
Tubal Metaplasia
Replacement of endocervical epithelium c epithelium of salpinges (somewhat unordered secretory, ciliated or peg cells) involving glands and surface epithelium, which may branch with hypercellularity of surrounding stroma
- low mits (vs endocervical AIS)
- may produce mucous grossly
- DES related?
- beware of tubal variant of adenocarcinoma in situ, which is ciliated
IHC: (+) p16 (can be problematic in the workup of glandular lesions in the cervix)
DDx: endocervical AIS

Tubal, Ciliated Metaplasia
Tuboendometrioid metaplasia
Mix of tubal and endometrioid types (vs single cell type in AIS)
- tubal cells can have ciilia or apical snouts
- lacks atypa, mits, and apoptotic bodies seen in AIS
IHC: p16 can be patchy (+), but not diffuse and strong as seen in AIS
Intestinal Metaplasia
Rare, but often assoc c AIS or invasive adenoca
Micro: has goblet cells w/o NE cells
- no nuclear atypia or mits
Diffuse Laminar Endocervical Glandular Hyperplasia
Rare, incidental finding in reproductive age women assoc c water/mucoid discharge
Micro: well-demarcated superficial lesion c glands lined by mucin-making columnar cells c rare mits
- can look reactive if inflam present
- should not be very atypical and no desmoplasia
Lobular Endocervical Glandular Hyperplasia
Rare, women of reproductive age mostly asymptomatic or c discharge, mass or abdominal discomfort
- may be assoc c Peutz-Jeghers
Micro: well-defined border of lesion c distinct lobular prolif of round variably-sized glands lined by simple mucin-producing columnar cells c bland basal nuclei usually in bottom 1/2 of cervical wall and sometimes surrounded by inflam
- can appear slightly atypical and have up to 2 mits / 10 hpf
IHC: (+) MUC6, HIK 1083, scattered chromogranin A and SYN
- neg: ER/PR, CEA
DDx: minimal deviation adenoca: has haphazard arrangement of glands c more variability in nuclei, and extends further through cervical wall and has desmoplastic rxn and sometimes vascular invasion (should not rely on IHC to make dx)
Microglandular hyperplasia
common, mostly in women of reproductive age (assoc c progesterone [OCPs and preg]), and up to 1/20 postmenopausal
- can grossly look like an erosion, polyp or friable raised area on the cervix
- may favor adenocarcinoma if large amt of tissue in bx (ie 3 blocks), lack of subnucleolar vacuoles, transition to other forms of endometrial adenoca, assoc c foamy stromal cells and + vimentin in cytoplasm
Micro: closely packed glands c variable shape and size c acute and chronic inflam c little intervening stroma, giving it a cribriform appearance
- epithelial lining is cuboidal to columnar c uniform nuclei c focal atypia, rare mits and produces mucin pools, possibly signet rings
- no nucleoli, can have different degrees of sq metaplasia
IHC: (+) p63 in reserve cell component, mucin
- neg: cytoplasmic vimentin (vs adenoca), p16 (though can be strongly but patchy positive, in which case doesn't co-localize MIB or cyclin E and not assoc c HPV infx), CD10
- CEA not helpful (neg in adenoca and microglandular hyperplasia)
Microglandular hyperplasia


Radiation-induced Atypia
Can persist up to years after tx
Micro: cytomegaly from nuclear and cytoplasmic swelling, cytoplasmic vacuoles, prominent nuclei, erosion and reactive changes
- stromal inflam c edema and necrosis
- atrophy s/p ~1 mo tx and hyalinized collagen
- stromal fibroblasts and endothelial cells c dilated BVs c thrombi can look scary
- decreased # endocervical glands, variable gland and cell size and shape,
Reactive / Reparative Squamous Atypia
Assoc c infx (candida, trich, chlamydia) or other chronic inflam
Micro: spongiosis, big nuclei, hyperchromasia or vesicular nuclei, prominent nucleoli, mild nulcear atypia and cytoplasmic halos, rare mits
- don't always see inflam in the bx
IHC: Ki67 (can have scattered [+] in upper 2/3 but not as much as SIL)
- neg: p16 (vs SIL)
Endocerical Polyps
Common b9 exophytic growths that arise in endocervical canal
- can be bumps or large polypoid masses that can protrude through cervical os
Micro: loose fibromyxoid stroma covered c mucous secreting endocervical cells
- usually assoc c inflam
B9 endocervical polyp

Inflam, reactive changes, and microglandular hyperplasia are common in endocervical polyps
Endocervicosis
Not common? Usually presents as dysmenorrhea / pelvic pain in younger women
- grossly mass in ant cervix
Micro: prolif of medium-sized glands c bland epithelium in outer 1/3 of cervix and paracervical ST
Epithelioid Trophoblastic Tumor (ETT)
- malignant gestational trophoblastic neoplasm (GTN) derived from the chorionic-type intermediate trophoblast
- rarest trophoblastic neoplasm, ETT typically occurs in reproductive-age females (mean age: 30 to 40 years) following term pregnancy, spontaneous abortion, or hydatidiform molar gestation.
-- The latency period between a gestational event and the development of ETT can range from months to years.
ETT frequently develops in the lower uterine segment or cervix (approximately 50% of cases)
- serum beta-hCG is usually mildly to moderately elevated (less than 2500 mIU/mL).
Micro: nodular growth of epithelioid cells with abundant eosinophilic cytoplasm and round nuclei with mild to moderate atypia.
-- see geographic necrosis, hyaline material surrounding neoplastic cells, and calcifications
IHC: (+) GATA3, alpha-inhibin, and p63,
- negative SALL4, ER, TTF1, p16, and PAX8
Px: Most cases of ETT are confined to the uterus and treated adequately with hysterectomy alone,
- up to 30% of cases develop metastases.
- FIGO stage remains the best predictor of outcome.
- lung is the most common metastatic site for ETT
DDX: Squamous cell carcinoma- Immunohistochemically, SCC should lack expression for HLA-G and H3D3B1, though it can stain positively for GATA3 and p63 which is a diagnostic pitfall. Some data support the use of CK18 (positive in ETT, negative in SCC). Most cervical SCC is HPV-driven, so diffuse p16 positivity would support the diagnosis of SCC of the cervix over ETT in the appropriate clinical context.
- Placental site stromal tumor: PSTT demonstrates a more infiltrative growth pattern, lacks the characteristic hyaline material of ETT, and is diffusely positive for hPL
Epithelioid trophoblastic tumor


Mesonephric Hyperplasia
Lobular, diffuse or ductal hyperplasia coming from mesonephric remnants
- MC is lobular, seen in 35 yo F and can extend deep, has large loosely organized lobules
- diffuse is 2nd MC, seen in 47 yo F, can form a mass or cause erosion, on micro is diffuse prolif of mesonephric tubules
- ductal type has prominent duct c papilalry tufting and minimal prolif of tubules
IHC: (+) EMA, calretinin, PAX-2, Bcl-2, androgen R, CD10 (luminal pattern), p16 focally, GATA-3 (strong vs patchy and weak in mesonephric adenoca)
DDx: mesonephric adenoca: looks pretty atypical and higher Ki67 (up to 15% vs 1-2% in mesonephric hyperplasia)
Nabothian cyst
Normal finding caused by obstruction of crypt opening by squamous epithelium thus dilating endocervical glands
- usually superificial but can go deep
- can cause acute/chronic cervicitis
Micro: uniform architecture, no stratification, mits, or atypia
- can cause reactive changes if rupture


Nabothian cysts
Placental Site Nodule
Well-circumscribed lesion of intermediate trophoblastic cells in hyalinized matrix
- has "pseudopods": irregular nests at the periphery of the nodule
- mits infrequent, binucleate cells common
IHC: similar to SCC, (+) CK, EMA, and p63, but is CD10, inhibin and HLA-G +, sometimes Mel-Cam+
- neg: p16
Tunnel clusters
Common, b9, incidental, assoc c multiple prior pregnancies, close to endocervical canal
- caused by localized prolif of endocervical glands c side channels growing out through them
Type A (non-cystic): well-circumscribed, pseudoinfiltrative, oval to pointed gland lumens, smaller cuboidal amphophilic or mucinous columnar, c big nuclei c mild nuclear atypia, florid glandular hyperplasia, and big nucleoli, minimal mits
- assoc c type B
Type B (cystic): larger glands c cystic dilation arranged in lobules, lined by bland flat to cuboidal cells c no mits
Micro: looks b9 c minimal atypia
IHC: (+)
- negative intracytoplasmic CEA
Tunnel clusters



Type B tunnel clusters
Rhabdomyosarcoma
Grape-like mass protruding from cervix to vagina
Types: embryonal (includes sarcoma botyroides), alveolar, anaplastic (pleomorphic)
- MC is embryonal
IHC: (+) focal desmin, muscle-specific actin, SMA, WT1, MyoD1
Tx: surgery and chemo in stage 1
Sarcoma botyroides
Rare form of embryonal rhabdomyosarcoma, in kids or early adolescents
- Grape-like mass protruding from vagina
Micro: rhabdomyoblasts at different stages of maturation
- cambium layer (dense group of cancer cells) under cervical epithelium
- might not look atypical in kiddas


A and B, Low-grade squamous intraepithelial lesion/cervical intraepithelial neoplasia 1 (LSIL/CIN1) with a mitotic figure (arrow) in the second third of the epithelium, showing only weak patchy staining with p16 immunostain. C and D, High-grade squamous intraepithelial lesion
(HSIL/CIN3) with strong, diffuse, ‘‘blocklike’’ p16 positivity (hematoxylin-eosin, original magnification x4 [A and C]; p16, original magnification x4 [B and D]).

A and B, Low-grade squamous intraepithelial lesion may show discordant (‘‘blocklike’’) p16 immunostaining pattern (hematoxylin-eosin, magnification x10 [A]; p16, magnification,x10 [B]).

A and B, High-grade squamous intraepithelial lesion with discordant, weak, and patchy p16 expression (hematoxylin-eosin, original magnification x20 [A]; p16, original x20 [B]).
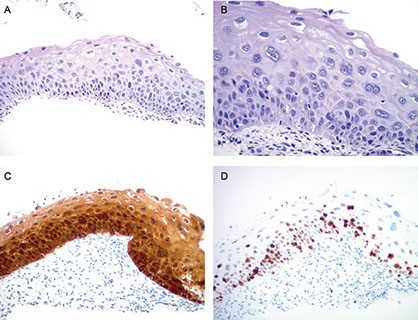
A and B, High-grade squamous intraepithelial lesion (CIN2). Lesion shows strong, blocklike p16 immunoreactivity (C) and increased Ki- 67 labeling extending up to two-thirds of the epithelial thickness in this case (D) (hematoxylin-eosin, original x4 [A] and x20 [B]; original magnification x10 [C and D]).
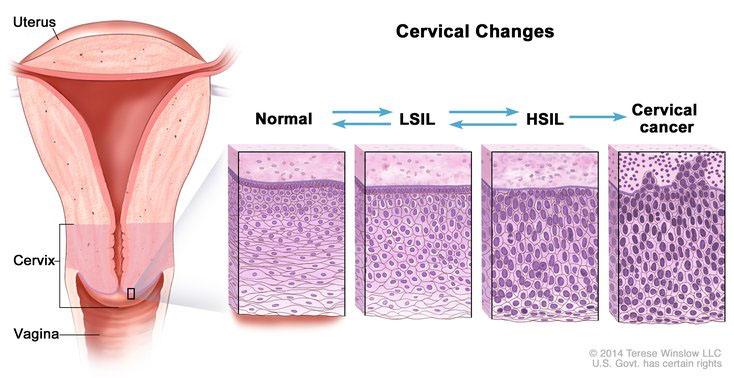
Squamous Intraepithalial Lesions (SIL)
2-tiered HPV-assoc SIL system based on LAST-project by CAP-ASCCP, which can then be broken down into more specific lesions
Risk factors: young at first intercourse, multiple sexual partners, cigarette smoking, immunocompromised, poor hygiene,
- HPV 6, 11, (low risk)
- HPV 16, 18, 31, 33, 35 (high risk)
- HPV vaccine available up to nonavalent (has 9 MC stands), and is approved for females from 9-26 yo
Low-Grade SIL (LGSIL or LSIL)
Has cytopathic HPV effect and mild-moderate cellular changes in the lower 1/3 of squamous epithelium
- assoc c HPV 6 and 11
- koilocytes formation assoc c viral E4 expression
-- productive infection (early and late gene expression) with non-integration of viral DNA
- early onset of juvenile respiratory papillomatosis
- ~3/5 regress, only ~1/1000 progress to carcinoma
Micro: koilocytes have marked nuclear variation (>3x variation), hyperchromasia, and irregular nuclear membranes ("raisinoid")
- cytoplasmic clearing is non-specific (can be reactive)
- basal layer loses its picket-fence pattern
IHC: 1/3 of LSIL are block p16+
High-Grade SIL (HGSIL or HSIL)
Includes CIN2+3
- CIN2 has mod-marked atypia in bottom 2/3 of squamous epithelium
- CIN3 has moderate-marked atypia in full thickness of the epithelium
- transforming infx c high-risk HPV types in high-grade intraepithelial lesions produce inc levels of E6 which binds p53 and E7 viral oncoprotein which binds and inactivates Rb causing upregulation of p16 by disruption of the negative-feedback loop
- integration of viral DNA
-- must be careful bc 2% of all b9 cases and 38% of low-grade lesions have shown positivity, and also up to 28% of high-grade lesions have been shown to be p16 negative
- p16 positivity in LG lesions may predict transformations to a HG lesions
Thin SIL [2] (historically called thin dysplasia):
Morphologically, these are immature intraepithelial lesions less than 10 cells thick. If a lesion is unequivocally SIL with significant immature abnormal basal proliferation or mitosis above the basal cells, it is designated as HSIL. If there is doubt about the nature of the proliferation (e.g., immature metaplasia versus SIL) then p16 staining can be used
Dysplasia extending into the endocervical glands (Gland-duct involvement??) [2]:
In general, grading of lesions extending into the
endocervical glands can be performed as with surface lesions. If the abnormal basal proliferation fills the gland with no or minimal evidence of maturation, this should be classified as high grade. However, it is important to be aware of the possibility of tangential sectioning of epithelial basal layers that may make accurate grading difficult or impossible.
IHC: (+) Ki67 in upper epithelial layers (although Ki67 can be expressed in the upper epithelial layers of LG lesions, and so should not be used to differentiate bwt the two)
- p16 (can be negative in low-risk HPV types but should be strongly positive in high-risk HPV types)
- p16 can help ddx bwt LSIL and HSIL
-- HSIL has strong diffuse block staining
- despite this, 1/3 of LSIL has p16 block positivity, and rare HSILs may not have block positivity (up to 30% are p16 negative)
ProExC, made of topoisomerase IIa (TOP2A) and Minichromosome Maintenance Protein 2 (MCM2) stains HG lesions and has reported 90% sensitivity and 79% PPV for HSIL
BIOMARKERS IN HPV-ASSOCIATED LOWER ANOGENITAL SQUAMOUS LESIONS [2]
1. p16 IHC is recommended when the H&E morphologic differential diagnosis is between precancer (–IN 2 or –IN 3) and a mimic of precancer (e.g., processes known to be not related to neoplastic risk such as immature squamous metaplasia, atrophy, reparative epithelial changes, tangential cutting).
2. If the pathologist is entertaining an H&E morphologic interpretation of –IN 2 (under the old terminology, which is a biologically equivocal lesion falling between the morphologic changes of HPV infection [low-grade lesion] and precancer), p16 IHC is recommended to help clarify the situation. Strong and diffuse block-positive p16 results support a categorization of precancer. Negative or non–block-positive staining strongly favors an interpretation of
low-grade disease or a non–HPV-associated pathology
3. p16 is recommended for use as an adjudication tool for cases in which there is a professional disagreement in histologic specimen interpretation, with the caveat that the differential diagnosis includes a precancerous lesion (–IN 2 or –IN 3).
4. WG4 recommends against the use of p16 IHC as a routine adjunct to histologic assessment of biopsy specimens with morphologic interpretations of negative, –IN 1, and –IN 3.
a. SPECIAL CIRCUMSTANCE: p16 IHC is recommended as an adjunct to morphologic assessment for biopsy specimens interpreted as
<=–IN 1 that are at high risk for missed high-grade disease, which is defined as a prior cytologic interpretation of HSIL, ASC-H, ASC-US/HPV-16þ, or AGC (NOS).

Pseudo-GDI? Cervical LSIL (CIN 1). Low-grade squamous intraepithelial lesion extends into an endocervical gland neck. When the full thickness of the abnormal epithelium is seen, the interpretation is straightforward. In areas with tangential sectioning (arrow), care must be taken not to overcall HSIL. Medium power, H&E [2]
Superficially Invasive Squamous Carcinoma
- aka Microinvasive squamous carcinoma
Defined as invasive carcinoma that [2]:
- is no a grossly visible lesion, AND
- Has an invasive depth of <3 mm from the basement membrane and the point of origin, AND
- Has a horizontal spread of <7 mm in maximal extent, AND
- Has been completely excised
Tongues of SCC breaking through the BM
- usually are better differentiated than in HSIL
- can be mimicked by inflam blurring the epithelial-stromal interface and tangentially cut endocervical glands, crushed epithelium and placental site nodules
- retraction artifact can be difficult to ddx from vascular invasion
Can be staged based on SGO or FIGO systems
- SGO: stromal invasion <3 mm below BM and doesn't invade blood vessels or lymph vessels
- FIGO: stage 1A: microscopic invasion; stage 1A1: invades <3 mm deep and <7 mm horizontally; stage IA2: invades >3 mm, < 5 mm deep and < 7 mm horizontally
- measured from the BM of HSIL to deepest invasive focus (should report depth of invasion in mm)
- in LEEPs or cold knife specimens also report # of foci, horizontal spread, presence / absence of vascular invasion, margin status
In cases of SISCCA, the following parameters should be included in the pathology report [1]:
- The presence or absence of LVI.
- The presence, number, and size of independent multifocal carcinomas (after excluding the possibility of a single carcinoma).
Micro: inc red cytoplasm, loss of nuclear polarity, sometimes keratin production
- usually present c florid HSIL in surface epithelium and deep endocervical glands, luminal necrosis in HSIL-involved glands, and aberrant intraepithelial squamous differentiation
- patterns of invasion: ragged/irreg cancer cell nests, epithelial reduplication, stromal epithelial nests, round tumor nests extending past glands, irreg nests c keratin production, stromal desmoplastic response, nests adjacent to medium-sized vessels
- can be graded by degree of differentiation as: intermediate > poorly > well
- can mimic clear cell ca c lots of glycogen
- worse px if contains mucin (confirm c IHC)

Figure 13. Cutaneous anogenital SISCCA: measurement of the depth of invasion. A, The depth of invasion is measured from the epithelialdermal junction of the adjacent-most superficial dermal papillae to the deepest point of invasion. This measurement is applicable whether the
surface epithelium is ulcerated or keratinized. This is the AJCCrecommended method of measuring vulvar squamous cell carcinomas in determining whether a tumor is stage T1a or T1b. B, Measurement for the thickness of the tumor when the epithelial surface is intact. If the
tumor is keratinized, the thickness of the tumor is measured from the granular cell layer to the deepest point of invasion. For squamous cell carcinomas, the convention is to measure from the bottom of the granular cell layer. If the epithelium is not keratinized, the thickness of
the tumor is measured from the surface of the tumor to the deepest point of invasion. C, Measurement for tumor thickness when the tumor is ulcerated. The tumor thickness is measured from the surface of the ulcerated tumor to the deepest point of invasion. For SCC, the depth of invasion is a more accurate measurement of the true depth of the tumor, as measured from the epithelial dermal junction of the adjacent dermal papillae to the deepest point of invasion. Reprinted with permission. Figure E.J. Wilkinson, 2007 From AJCC Cancer Staging Manual, 6th ed. New York, NY: Lippincott, Williams & Wilkins; 2002. [2]
Squamous cell carcinoma
Precurson lesion is cervical intraepithelial neoplasia
- pts usually have postcoital bleeding and abnormal Paps before turn to cancer
- MC high-risk HPV is HPV-16
IHC: (+) AE1/AE3, CEA, CAM5.2, CK5/6, p63
- neg: vimentin
Px: depends on size of tumor,
- poor px it is small cell variant, presence of HPV18 and inc cell prolif index, usually found c SCC or AC
- having lots of mature eosinophils assoc c the tumor is a good prognostic indicator
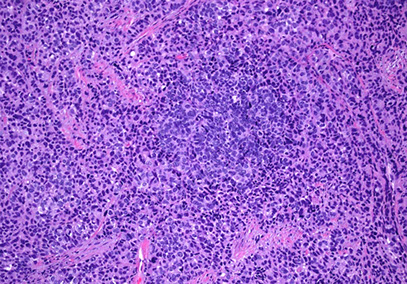
Small cell carcinoma
asdf
Small cell carcinoma of the cervix, assoc c HPV 18, CHR+
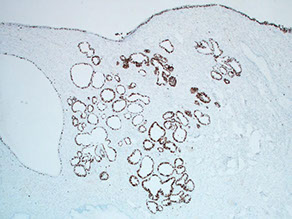
Stratified Mucin-Producing Intraepithelial lesion (SMILE)
carcinoma with cells that appear to by a hybrid of squames and mucinous columnars
SMILE IHC: Ki67 high, block p16, MUC+, neg IMP3 and CK14-
SMILE

Adenocarcinoma in Situ (AIS)
Seen in rep age women (avg 30 yo)
- assoc c High Risk HPV, esp type 16 and 18
- many that present c AIS have cervical IN (CIN), or squamous intraepithelial neoplasia (SIN), or hx CIN/SIL
- seen close to SC junction, can be multifocal
- involvement of deep glands more obvious, but can involve superficial glands
Micro: cells crowded or stratified, large nuclei c dark coarse chromatin, little nucleoli, var stratification, inc (freq) mits; apoptotic bodies seen
- usually an abrupt transition from normal epithelium (clear demarcation of different glands)
- several subtypes: endocervical (MC), endometrioid, intestinal (gastric2), tubual, adenosquamous, clear cell
- calling something dysplastic glands not very good
- IHC: (+) p16 (strong, diffuse), Ki67 (>30%),
-- negative: nuclear PAX2 (loss of expression), ER/PR
- HPV ISH helps to show is HPV-driven process
DDx: Benign endometrial epithelium (reactive changes, tuboendometrial metaplasia, proliferative endometrial epithelium, endometrium / cervical endometriosis) are usually p16 neg although can have patchy p16 immunoreactivity (not diffuse tho)
- Paired box 2 (PAX2) has diffuse positivity in b9 cervical tissue (including tubal metaplasia, lobular endocervical glandular hyperplasia, and b9 mesonephric proliferations)
- b9 conditions have low Ki67
- ER and vimentin show diffuse positivity in endometrial epithelium and CD10 highlights endometrial stroma
NOTE: mesonephric AC and gastric-type AC are NOT assoc c high-risk HPV and therefore lack p16 immunoreactivity in most cases
- mesonephric AC usually positie for CD10, PAX8, vimentin and CEA, and can rarely have diffuse p16+
Clear cell AIS
cuboid, polygonal or flat cells c clear to red cytoplasm, big nuclei, inc mits
Endocervical AIS
moderate cytoplasm c dec mucin (vs normal endometrial gland)
Endometrioid AIS
no mucin, lots of pseudostritification, big nuclei, inc mits
Intestinal AIS
has goblet cells c large round cytoplasmic vacuole
Endocervical AIS


p16
Ki67


A and B, Small fragments of endocervical adenocarcinoma in situ (AIS) in an endocervical curettage with nuclear atypia, hyperchromasia, crowding, and frequent apical mitoses (arrows). P16 immunostaining is strongly and diffusely positive (C) and Ki-67 shows increased proliferation index (D) (hematoxylin-eosin, original x20 [A and B]; magnification x20 [C and D]).
Invasive endocervical AC


A and B, Endometriosis mimicking endocervical adenocarcinoma in situ (AIS) in a cervical biopsy. P16 immunostaining is patchy in the endometrial glandular epithelium (C) and CD10 immunostain highlights endometrial stroma (D) (hematoxylin-eosin, original magnifications34 [A] and x10 [B]; original magnification x20 [C and D]). [1]
Superficially Invasive Adenocarcinoma
- aka Microinvasive adenocarcinoma
No standardized definition
- FIGO IA1 <0.3 cm invasion of glands
- FIGO IA2 bwt 0.3-0.5 cm deep up to 0.7 cm wide
- lymphatic and vascular invasion do not alter stage
Invasive Adenocarcinoma
Cancerous glands invade stroma
- glands are architecturally complex and pleomorphic, have irreg contours
- normal glands can extend for up to 0.9 cm (should compare with adjacent glands)
- Most are assoc c HPV infx, esp HPV 16 and 18
- primary AC of cervix is rare (5-15% of all cervical ca)
- incidence is increasing in Jewish women and young women
- several subtypes:
Endocervical Adenoca, Usual Type
4/5 cervical adenocas, usually mod differentiation c not very mucinous glands
- high-assoc c high-risk HPV
- have complex growth pattern (confluent glands, cribriform or papillary)
- can also show reactive / desmoplastic stroma, deep glands, glands adjacent to thick walls, and lymphatic invasion
Villoglandular Adnoca
Uncommon, on micro almost all tumor is tall, thin papillae c mildly atypical epithelium
- usually premenopausal pts c abnormal bleeding and irregular Paps
- may have better px
Cervical AC with Gastric Differentiation
Non-HPV related subtype of cervical AC that shows morphologic similarity to gastric pyloric-type and pancreatobiliary AC
- is aggressive if poorly differentiated, and usually has mets to ovary, and lymph nodes at presentation
IHC: (+) CK7, CEA, EMA, CK20 (focal), CDX2, markers of pyloric-type mucin (MUC6 and H1K1083)
- may have focal PAX8 positivity
- p53 usually mutated, and may have an abnormal staining pattern
- neg: p16 (negative or focally positive) and vimentin
Adenoma malignum may be considered a well-diff form of cervical AC with gastric differentiation

A, Endocervical adenocarcinoma, usual type. P16 immunostaining is strongly and diffusely positive (B), carcinoembryonic antigen (CEA, monoclonal) shows cytoplasmic and membranous staining (C), and estrogen receptor (ER) shows only focal weak positivity (D). Vimentin immunostaining is negative in the tumor cells (not shown) (hematoxylin-eosin, original magnification x10 [A]; original magnification x10 [B through D]).
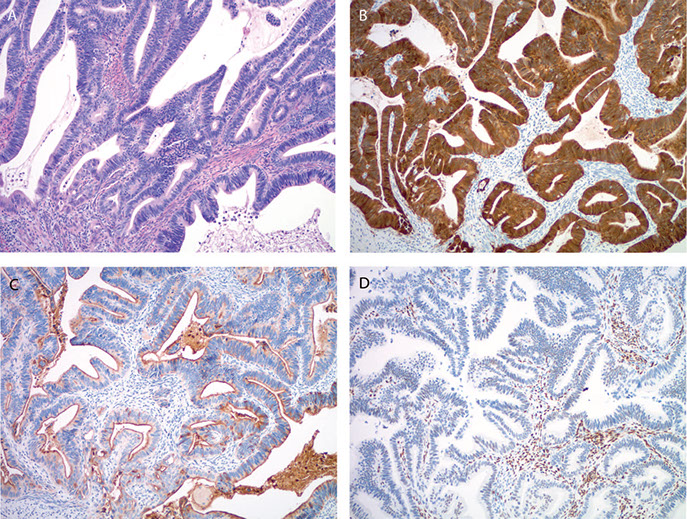
A, Gastric-type endocervical adenocarcinoma with markedly atypical, mucinous glands infiltrating the cervical stroma. Paired box protein 8 (PAX8) immunostain shows focal, weak nuclear positivity (B) and carcinoembryonic antigen (CEA, monoclonal) shows diffuse positivity (C). Focal staining may be observed with caudal type homeobox 2 (CDX2) (D). P16 typically shows negativity (E), while p53 shows strong diffuse immunoreactivity (F) (hematoxylin-eosin, original magnification x10 [A]; original magnification x10 [B through F]).


Adenoma Malignum
- aka minimal deviation adenocarcinoma
- Assoc c Peutz-Jeghers syndrome (~1/20)
- well-differentiated form of cervical AC with gastric differentiation (?) [1]
- non-HPV related subtype of cervical AC
Grossly has friable mucosa or mass
Micro: pleomorphic neoplastic glands are lined by mucin-producing, columnar cells with basally located nuclei. - glands are variable in shape and size and sometimes surrounded by desmoplastic response
- sometimes vascular / lymph invasion
- rare mits, no HPV assoc
- IHC: (+) CEA, p53
-- neg: PAX2 (loss of expression)

Adenoma malignum

Mucinous Adenocarcinoma, Gastric Type
- Micro: celsl c pale or red cytoplasm c distinct cell borders, good amt of cytologic atypia
- no HPV assoc
- aggressive
Mucinous Adnoca, Intestinal type
Carcinoma c goblet or Paneth cells
- HPV sometimes assoc
- IHC: (+) CK7/20, CDX2, CEA (focal), p16 (focal or neg)
Mucinous Adnoca, Signet-Ring Cell type
Rare
- must be careful bc usually signet ring cells seen in poorly diff adenoca or adenosquamousca
Mucinous Adnoca, Colloid type
super rare, has lots of mucin in stroma
Endometrioid Adenocarcinoma
Similar histology as same ca seen in endometrium
- sq diff less common
- favor cervical origin if it is in situ (AIS), lack of complex endometrial hyperplasia if endometrial tissue present, and if IHC (+) CEA and p16 c neg vimentin and ER
Clear Cell Carcinoma (CCC)
Rare in cervix, possible DES or HPV connection
- histologically identical to CCCs of endometrium and ovary: clear to red cytoplasm, polyhedral, square or flat cells in papillary, tubulocystic or solid gland arrangement
- clear cytoplasm 2/2 glycogen
Serous carcinoma
Rare, same as other serous ca's, bimodal age distr
- cervix can be ulcerated or have mass and can present c bleeding or discharge
- Micro: lots of atypia and mits, psammoma bodies, invasion to lymph
- IHC: (+)p53, CEA, HPV
- Px: recur if advanced stage (>FIGO I), >2 cm size, >1 cm invasion, LN mets
Adenocsquamous Ca (Adosqca)
Mix of endometrioid adoca c sq diff
- px similar to adoca and sqca stage for stage
Glassy Cell Carcinoma
Poorly diff variant of adenosquamous carcinoma in younger females that grows fast and is more aggressive
Grossly has a barrel-shaped uterus (which is elongated and thickened
- Micro: nests/sheets of big cells c big nucleoli and lots o mits. ground glass cytoplasm, lots of inflam c eos and plasma cells
- IHC: (+) p53, 34BE12, CAM5.2, MUC1/2. CEA, var ER/PR and Her2
- should r/o poorly diff sqca and lymepi-like ca
Glassy cell ca (variant of adenosquamous ca)
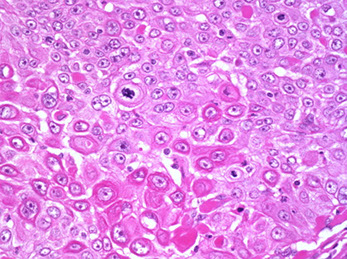
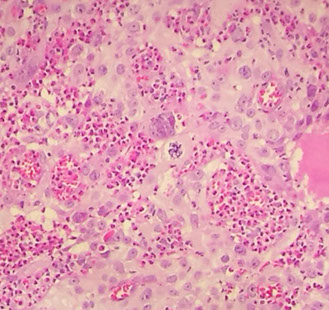
Adenoid Basal Ca (Epithelioma)
Older women (postmen) can present c bleeding, usually no tumor grossly
Micro: Uniform, bland, basaloid cells in nests c peripheral palisading, no desmoplastic rxn c small uniform cells that can be spindly, can have glycogen inclusion
- usually assoc c high grade Sq Intraepithelial Lesion
- no necrosis, perineural invasion
- IHC: (+) CAM5.2, CK7, EMA, CEA, p53. p63
- Px: typically benign (no recurrence, mets or deaths)
- should r/o adenoid basal hyperplasia, ectopic prostate, adenoid cystic ca, basaloid sq ca
Adenoid Cystic Carcinoma
Similar to same tumor of salivary glands, old women (postmen) presenting c bleeding, can form mass
- cribriform c red secretion, mits var, usually some necrosis,
- IHC: (+) p63, CD10, palponin, sma
- Px: aggressive
Microcystic Adenocarcinma
Present c bleeding, var age range
- micro: Lots of cysts, must have regoin c conventional endocervical adenoca
-can look b9, like nabothian cysts or type B tunnel clusters
Mesonephric Adenoca / Malignant Mixed Mesonephric Tumors
Derived from remnants of mesonephric duct, has sarcoma in addition to adenoca, var histologic patterns, MC are ductal and tubular c columnar-cuboidal cells mod pleomorphism and var mits
- IHC: (+) pan CK, CK7, CAM 5.2. EMA, calretinin, vimentin, CD10
- neg CEA, CK20, ER/PR
- must r/o diffuse mesophric hyperplasia, endometrioid adenoca, MMMT,
Basaloid Squamous Carcinoma
Under-recognized subtype of SCC that has lots of big mitotically active islands c dark nuclei and peripheral palisading
Px: largely unknown
Lymphoepithelioma-Like Carcinoma
Rare, made of nests or trabeculae of undifferentiated cells surrounded by inflam w/o keratinization or intracellular bridging
- large polygonal tumor cells c indistinct cell borders c vesicular chromatin and prominent nucleoli and red cytoplasm
- need a well-demarcated border to dx
Px: favorable, rarely mets to LNs
Neuroendocrine Carcinoma
Micro: apoptotic bodies, nuclear molding and crush artifact, more common than in uterus
- can have large geographic necrosis and apoptosis if large cell
- assoc c HPV
IHC: (+) SYN, CD56, CHR, CK5/6 (sometimes)
Papillary Squamous Cell Carcinoma
Difficult in dx bc don't have enough tissue to see stroma to tell if invades, usually requiring LEEP or cold knife
- may be more squamous or basaloid
Px: tend to have late recurrences
Spindle Cell (Sarcomatoid) Carcinoma
Rare SCC subtype c spindle cell features
Micro: MNGCs that can appear osteoclastic
IHC: (+) keratin and vimentin in spindle cell areas, EMA
Px: advanced dz and recurrence usually fatal
Transitional Cell Carcinoma
IHC: (+) Glypican 3, p40, GATA-3, PAX-8 (all are also seen in SCC)
- neg: CK7/20
Verrucous Carcinoma
Very rare, broad papillae on surface c pushing pattern of invasion c bulbous nests
- cannot be dx'd on bx bc need to eval whole lesion to see if invasive or if atypia present
Micro: lots of cytoplasm and little to no atypia
Px: high-local recurrence (1/2) but no mets
References
1. Buza N. Immunohistochemistry in the Gynecologic Tract. Arch Pathol Lab Med 2017; 141:1052-1071
2. Darragh et al. CAP-ASCCP LAST Project. Arch Pathol Lab Med; vol 136, Oct 2012
