Antimicrobials
Antibiotics
Antimicrobial Sensitivity Testing
Antimycobacterials
Antifungals
Antivirals
Antibiotics
General Mechanisms of Action
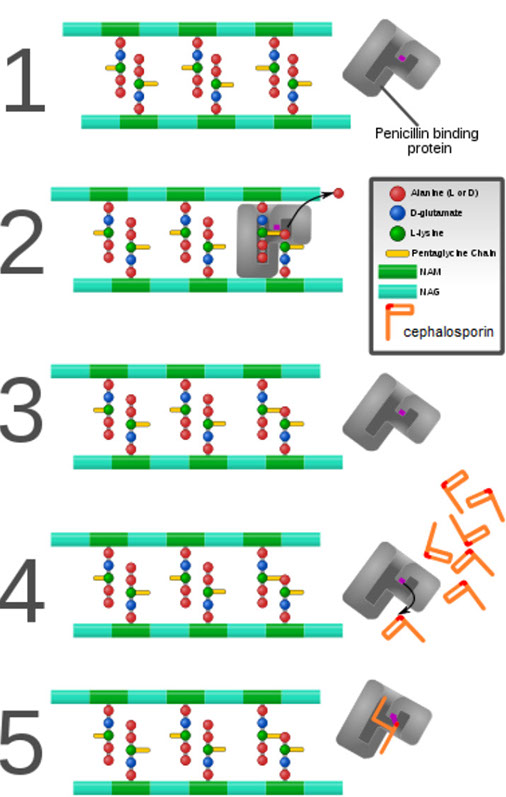
1. Block cell wall synthesis by inhibiting peptidoglycan cross-linking
- penicillin, methicillin, ampicillin, piperacillin, cephalosporins, aztreonam, imipenem
2. Block peptidogltcan synthesis
- bacitracin, vancomycin
3. Block nucleotide synthesis
- sulfonamides, trimethoprim
4. Block DNA topoisomerases
- fluoroquinolones
5. Block mRNA synthesis
- Rifampin
6. Damage DNA
- metronidazole
7. Block protein synthesis at 50S ribosomal subunit
- Cloramphenicol, macrolides, clindamycin, streptogramins (quinupristin, dalfopristin), linezolid
8. Block protein snth at 30S ribosomal subunit
- aminoglycosides, tetracyclines
Penicillin
Penicillin G (IV formn), Penicillin V (oral), Prototype B-lactam antibiotics
Mech: 1) Binds penicillin-binding proteins (PBPs)
- PBPs are transpeptidases that are involved in the final stages of the synth of peptidoglycan (a major component of cell walls)
2) Blocks transpeptidase cross-linking of cell wall
3) Activates autolytic enzymes
Clinical use: Bactericidal for gram-pos cocci (grp A and B Streptococcus), gram-pos rods (Claustridium and Listeria), gram-neg cocci (Neisseria), spirochetes.
- Not penicillinase resistant
Tox: Hypersensitivity rxns, hemolytic anemia
Methicillin, nafcillin, dicloxacillin (penicillinase- resistant penicillins)
Mech: same as penicillin; narrow spectrum; penicillinase resistant bc of bulkier R group
*** use Naf (nafcillin) for staph ***
Clinical use: S aureus (except MRSA; resistant bc of altered PBP target site)
Tox: hypersensitivity rxns, methicillin can cause interstitial nephritis
Ampicillin, amoxicillin (aminopenicillins)
Mech: same as penicillin; wider-spectrum; penicillinase sensitive (also combined c clavulanic acid)
*** amOxicillin has great Oral bioavailabilitty than ampicillin ***
Clinical use: Extended-spectrum penicillin - certain gram-pos bacteria and gram-neg rods (Haemophilus influenzae, E coli, Listeria monocytogenes, Proteus mirabilis, Salmonella, enterococci) -- good for UTIs
** Coverage: ampicillin/amoxicillin HELPS get rid enterococci ***
Tox: HS rxns, ampicillin rash (esp occurs when ampicillin given during infectious mono [EBV]), and pseudomembranous colitis (esp ampicillin)
Ticarcillin, carbenicillin, piperacillin (antipseudomonals, aka the carboxypenicillins)
Mech: Same as penicillin; extended spectrum
Clinical use: Pseudomonas spp and gram-neg rods; susceptible to penicillinases -- used with clavulinic acid B-lactamase inhibitor
Penicillinase (B-lactamase) inhibitors: Clavulanic Acid, Avibactam, Sulbactam, Tazobactam
*** CAST ***
- have little activity on their own
- inhibit B-lactamases - used in combo c B-lactams to inc activity
-- Amoxicillin + clavulanic acid = Augmentin
-- Ampicillin + sulbactam = Unasyn
-- Piperacillin + tazobactam = Zosyn
-- Ceftazidime + avibactam = Avycaz
*** TCP: Takes Care of Pseudomonas ***
Tox: HS
Cephalosporins
Mech: B-lactam drugs that inhibit cell wall synthesis but are less susceptible to penicillinases. Bactericidal
Cefazolin, Cephalexin, Cephalothin (!st generation, narrow spectrum)
- used for gram-pos cocci (Staph and Strep, except MRSA), Proteus mirabilis, E coli, Klebsiella pneumoniae
*** 1st generation = PEcK ***
Cefoxitin, Cefotetan, Cefaclor, Cefuroxime (2nd generation, Expanded spectrum)
- adds some gram-neg and anaerobic coverage; used for gram-pos cocci, Haemophilus influenzae, Enterobacter aerogenes, Neisseria spp, Proteus mirabilis, E coli, Klebsiella pneumoniae
*** 2nd generation = HEN PEcKS ***
Ceftriaxone, Cefixime, Cefotaxime, Ceftazidime (3rd generation, Broad spectrum)
-- more Gram-neg activity (but less Gram-pos and anaerobic activity); serious gram-neg infx resistant to other B-lactams; meningitis (most penetrate blood-brain barrier)
-- Ceftrixone -- meningitis and gonorrhea
-- Ceftazidime -- Pseudomonas
Cefepime (4th generation, Extended spectrum)
Very good Gram neg activity, better B-lactamase activity; Inc activity against Pseudomonas and gram-pos bugs
Ceftaroline (4+ generation)
Spectrum activity Gram-pos (including MRSA) and Gram-neg bacteria (not ESBL)
Tox: HS, vit K deficiency
- cross HS c penicillins in <10% of pts; inc nephrotoxicity of aminoglycosides
- disulfiram-like rxn c alcohol use (in cephalosporins c a methylthiotetrazole group like cefamandole)
Resistance: B-lactamases, including extended spectrum B-lactamases; altered penicillin binding protein (PBP)
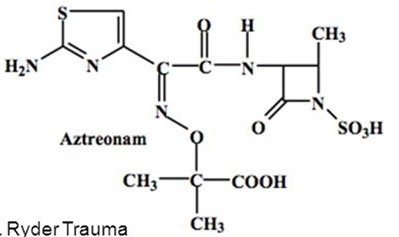
Aztreonam
A monobactam resistant to B-lactamases
- inhibits cell wall synth (binds to PBP3)
- synergic c aminoglycosides
- no cross allergenicity c penicillins
Clinical use: Gram-neg rods only -- no activity against gram-pos or anaerobes
- for penicillin allergy pts, and pts c renal insufficiency who cannot tolerate aminoglycosides
Tox: usually non-toxic; maybe GI upset
Imipenem/cilastatin, meropenem, Ertapenem (Carbapenems)
Broadest spectrum activity Gram-pos (except MRSA) and Gram-neg bacteria including anaerobes
Mech: Imipenem is a broad-spectrum B-lactamase resistant carbapenem; always given with cilastatin (inhibitor of renal dehydropeptidase I) to dec inactivation of drug in renal tubules
Clinical use: gram-pos cocci, gram-neg rods, and anaerobes; wide-spectrum, but the side effects limit use to life-threatening infx or after other drugs fail
- meropenem, though, has a dec risk of seizures and is stable c dihydropeptidase I
Tox: GI distress, skin rash, CNS toxicity (seizures) at high plasma levels
Vancomycin
Mehc: inhibits cell wall mucopeptide formation by binding D-ala D-ala portion of cell wall precursors. Bactericidal
Clinical use: Gram-pos only - for serious multidrug -resistant organisms, such as S aureus, enterococci and Clostridium difficile (oral dose for seuodmembranous colitis)
Tox: Nephrotoxic, Ototoxic, Thrombophlebitis, flushing (red man syndrome, can be prevented by slow infusion and antihistamines) -- generally does NOT have many problems
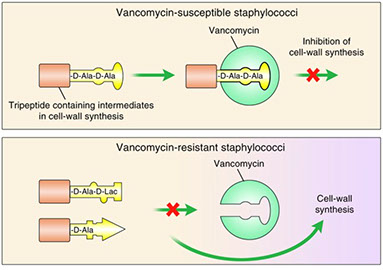
Resistance: Amino acid change of D-ala D-ala to D-ala D-lac
*** Pay back 2 D-ala (dollars) for vandalizing ***
- may inc cell-wall thickness
Polymyxin B and E (aka Colistin)
Mech: bind to lipopolysaccharide in outer membrane of Gram-neg bacteria, disrupting the inner and outer membranes, possibly c a detergent-like mech of action
- produced in nature by gram-pos bacteria such as Paenibacillus polymyxa
Clinical Use: multiple-drug resistant Pseudomonas aerugenosa or carbapenemase-producing Enterobactericeae
- are not absorbed through GI tract, so only given orally to treat gastrointestinal infx
- can be given as drops for otitis media
- can be given parenteral
Tox: neurotoxic, nephrotoxic, used as a last resort
Protein Synthesis Inhibitors
*** Buy AT 30; CCEL (sell) at 50 ***
- target small bacterial ribosome (70S made of 30S and 50S subunits), leaving human chromosome (80S) unaffected
30S inhibitors
A = Aminoglycosides (bactericidal)
T = Tetracclines (bacteriostatic)
50S inhibitors
C = Chloramphenicol and Clindamycin (bacteristatic)
E = Erythromycin (macrolides) [bacteriostatic]
L = Linezolid (variable)
Gentamicin, Neomycin, Amikacin, Tobramycin (Aminoglycosides)
*** "Mean" GNATS canNOT kill anaerobes ***
Mech: bactericidal; inhibit formation of initiation complex and cause misreading of mRNA
- need O2 for uptake, thus are not effective against anaerobes
Clinical use: severe gram-neg rod infx; synergistic c B-lactamse antibiotics against S aureus and Enterococcus
- neomycin for bowel surgery
Tox: Nephrotoxic (esp if used c cephalosporins), Ototoxic (esp if used c loop diuretics), Teratogen
Resistance: Transferase enzymes inactivate the drug by acetylation, phosphorylation or adenylation
- also may alter membrane permeability
- modification (methylation) of ribosomal target
Tetracycline, Doxycycline, Demeclocycline, Minocycline (Tetracyclines)
*** Demeclocycline -- ADH antoagonist; acts as Diuretic in SIADH ***
Glycylcyclines (Tigecycline) - broader-spectrum gram-neg and anti-staphylococcal activity than other tetracyclines
Mech: Bacteriostatic. Binds to 30S and prevents attachment of aminoacyl-tRNA; limited CNS penetration
- doxyxyxline fecally eliminated and can be used in pts c renal failure
-- must NOT take c milk, antacids, or iron-containing preparations bc divalent cations inhibit absorption in gut
Clinical use: Borrelia burgdorferi, M pneumoniae. Drugs ability to accumulate intracellularly makes it very effective against Rickettsia and Chlamydia
Tox: GI distress, discoloration of teeth and inhibition of bone growth in kiddos, photosensitivity.
- Contraindicated in pregnancy
Resistance: Dec uptake into cell or inc efflux out of cell by plasmid-encoded transport pumps
- modification (methylation) of ribosomal target
- tetracycline-modifying enzymes
Erythromycin, Azithromycin, Clarithromycin (Macrolides)
Inhibit protein synth by blocking translocation (macroSlides); bind to 23S rRNA of the 50S ribosomal subunit. Bacteriostatic
Clinical use: Atypical pneumonias (Mycoplasma, Chlamydia, Legionella), URIs, STDs, gram-pos cocci (streptococcal infx in pts allergic to peniciilin) and Neisseria
Tox: prolonged QT interval (esp erythromycin), GI problems, acute cholestatic hepatitis, eosinophilia, skin rash,
Resistance: Methylaion of 23S rRNA binding site
- efflux pumps (macrolides and ketolides)
Chloramphenicol
Mech: blocks eptide bond formation at 50S ribosomal subunit. Bacteriostatic
Clinical use: Meningitis (Haemophilus influenzae, Neisseria meningitidis, Streptococcus pneumonae); conservative use 2/2 tox but often still used in developing countries 2/2 low cost
Tox: Anemia (dose-dependent), aplastic anemia (dose dependent), gray baby syndrome (in premature infants bc lack liver UDP-glucoronyl transferase)
Resistance: Plasmid-encoded acetyltransferase inactivates drug
Clindamycin
Mech: Blocks peptide bond formation at 50S ribosomal subunit. Bacteriostatic
Clinical use: anaerobe infx (Bacteroides fragilis, Clostridium perfringens) in aspiration pneumonia or lung abscesses
Tox: Pseudomembranous colitis
Sulfamethoxazole (SMX), Sulfisoxazole, Sulfadiazine (Sulfonamides)
Mech: PABA antimetabolites inhibit dihydropteroate synthetase. Bacteriostatic
Clinical use: Gram-pos, gram-neg, Nocardia, Chlamydia. Triple sulfas or SMX for simple UTI
Tox; HS, hemolysis if G6PD deficient, nephrotoxic (tubulointerstitial nephritis), photosensitivity, kernicterus in infants, displaces other drugs from albumin (warfarin)
Resistance: Altered enzyme (bacterial dihydropteroate synthetase), dec uptake or inc PABA synth
Trimethoprim
Mech: Inhibits bacterial dihydrofolate reductase. Bacteriostatic
Clinical use: used in combo c sulonamides (TMP-SMX) causing sequential block of folate synth. Combo for UTIs, Shigella, Salmonella, Pneumocystic jiroveci pneumonia, Burkholderia cepacia and Stenotrophomonas maltophilia
Tox: Megaloblastic anemia, leukopenia, granulocytopenia (can be alleviated c supplemental folinic acid; the leucovorin rescue)
Ciprofloxacin, Norfloxacin, Levofloxacin, Ofloxacin, Sparfloxacin, Moxifloxacin, Gatifloxacin, Enoxacin (Fluoroquinolones) and Nalidixic acid (a quinolone)
Mech: inhibits DNA gyrase (topoisomerase II). Bactericidal. Must not be taken c antacids
Clinical use: gram-neg rods or urinary and GI tracts (including Pseudomonas), Neisseria, some gram-pos organisms
Tox: Tendonitis and tendon rupture in adults, leg cramps and myalgias in kiddos; contraindicated in pregs and kiddos bc damages cartilage
Resistance: chromosome-encoded mutation in DNA gyrase and/or DNA topoisomerase
Metronidazole
Mech: forms free radical toxic metabolites in bacterial cell that damage DNA. Bactericidal, and antiprotozoal
Clinical use: treats Giardia, Entamoeba, Trichomonas, Gardnerella vaginalis, Anaerobes (Bacteroides and C difficile)
- used c bismuth and amoxicillin (or tetracycline) for "triple therapy" against H pylori
Tox: disulfiram-like rxn c alcohol, headache, metallic taste






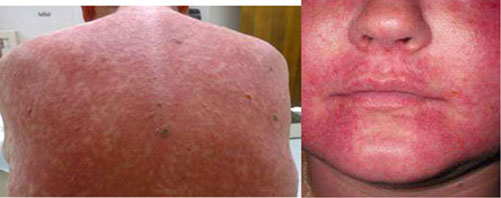
Red man syndrome


Tetracyclines


Sulfonamides




The primary target of the fluoroquinolones is DNA gyrase, which relaxes and supercoils bacterial DNA during replication by cutting the DNA, passing it across another strand, and resealing it. The secondary target is topoisomerase IV, which unlinks the newly replicated DNA strand from the parent strand

Colistin

Antimicrobial Susceptibility Testing
Qualitative
Kirby Bauer disc diffusion -- results interpreted as susceptible, intermediate, or resistant
Quantitative
Broth or Agar dilution -- results interpreted as lowest concentration of antibiotic that inhibits bacterial growth (MIC)
Gradient Diffusion
Set up like a qualitative test, read like a quantitative test
Not everything that is tested is reported -- report based on clinical efficacy, prevalence of resistance, minimizing emergence of resistance and cost
Reported as determined by cutoffs set by the CLSI using epidemiological and pharmacodynamic data:
Susceptible (S) -- susceptible to usual doses in accessible sites; does not mean in the middle of an abscess
Intermediate (I) -- susceptible at inc doses or where the drug is concentrated; usually means a drug to use in urine or if no better choice is available
Resistant (R) -- resistant to the usual doses
Disk Diffusion
Bacteria spread in a confluent lawn on plate
- antibiotic disk added to lawn and diffuses from disk into agar
- a zone of inhibition is formed around the disk
- size of the zone is correlated c the MIC
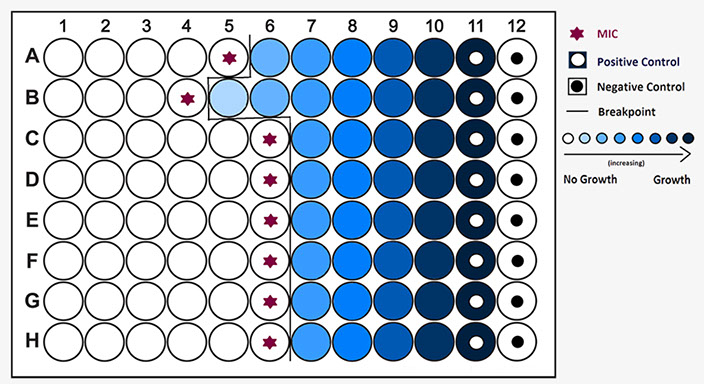
Minimum Inhibitory Concentration (MIC) Testing
Liquid or solid media c varying dilutions of antibiotic
- lowest concentration that inhibits growth
Gradient Diffusion (Etest)
Bacteria spread in confluent lawn on plate
- antibiotic gradient strip added to lawn and antibiotic diffuses into agar
- an ellipse of inhibition is formed around the strip
- where organism growth intersects the plastic strip = MIC
B-lactamase Detection
- filter paper impregnated c chromogenic cephalosporin (nitrocefin)
- results in minutes
- limited applications
-- detects resistance to ampicillin/penicillin (not cephalosporins) in Haemophilus, Neisseria gonorrhoeae, Moraxella catarrhalis, Enterococcus, and Staphylococcus
Resistance Detection Issues
S. pneumoniae
- overall resistance increasing
- Penicillin resistance -- altered PBPs
-- Intermediate resistance - 30-40%
-- High level resistance -- 5-15%
Testing isolates
-- oxacillin screen - susceptible >20 mm
-- confirm results <19 mm c MIC testing
- E-test or broth microdilution
Separate interpretive standards for penicillin and cephalosporins for:
- meningitis (S<0.06, R>0.12)
- Non-meningitis parenteral (S<2, I=4, R>8)
- Oral therapy (S<0.06, I=0.12-1, R>2)
S. aureus
Methicillin-Resistant S Aureus (MRSA)
S aureus is a major healthcare and community-acquired organism, causes ~1/5 cases if bacteremia in the US/Canada
- MRSA first described in 1961; first penicillinase- resistant semi-synthetic (methicillin) introduced in 1960
-- oxacillin resistance mediated by acquisition of mecA gene -- codes for altered PBP --> PBP2a
MRSA should be considered resistant to ALL penicillins, cephalosporins, B-lactam / B-lactamase inhibitor combinations, carbapenems
Heterogeneous resistance -- MRSA grows more slowly, MRSA growth enhanced by NaCl
Detection -- oxacillin screening agar, cefoxitin disk test, PBP2a antigen, molecular testing, chromogenic media
Oxacillin Screening Agar
- 6 ug/mL oxacillin, Mueller-Hinton agar supplemented c 4% NaCl, incubated 24 hrs before reading; requires separate piece of media, 24h reporting delay if not performed on all isolates
Cefoxitin Disk Screen
30 ug cefoxitin disk; Report zone ≤19mm as oxacillin resistant; Also works for coagulase(-) staph, zone ≤24mm; Relatively inexpensive; Requires a separate plate, but may be combined with D-test; Now incorporated into automated testing systems
PBP 2a Antigen
Rapid antigen test for PBP2a; gene product of mecA
– Rapid but expensive; May require overnight induction for best sensitivity
Molecular Testing
Used both for screening and culture confirmation
– False-positives due to gene deletions outside the detection amplicon
Chromogenic Media
Selective / differential media specific for MRSA; no AST required
Tx for MRSA: Vancomycin
Vancomycin Intermediate S. aureus (VISA)
Relatively rare; Difficult to detect
– Cannot use disk diffusion testing for vancomycin
• Decreased fitness; assoc c poor clinical response
Vancomycin Resistant S aureus (VRSA)
• Vancomycin MIC >8
• Acquisition of vanA cluster from Enterococcus
• Typically very high MIC, no loss of fitness
• May be missed by automated systems
• Vancomycin Screening Agar required for sensitive detection
Other vancomycin issues -- recent data suggests that S. aureus c MIC of 2 vs vancomycin (still S by CLSI breakpoints) have poorer outcomes when tx'd c vancomycin
- perform and report MIC or E-test on systemic MRSA isolates
Inducible clindamycin resistance
- macrolide resistance -- methylation of ribosome (erm gene - constitutive or inducible)
- efflux (msrA gene)
- staph resistant to both erythromycin and clindamycin = constitutive erm gene
- staph with an inducible erm gene that's treated c clindamycin can be easily induced to become resistant due to cross-resistance c erythromycin, leading to treatment failure
Until recently conventional automated AST did not detect inducible resistance well
• Test all Staph resistant to erythromycin and susceptible to clindamycin to determine if
– Inducible erm expression – clindamycin will become
resistant, cannot be used for therapy
– Efflux (msrA expression) – clindamycin can be used for therapy
• Perform D test
• 2 ug clindamycin disk; 15 ug erythromycin disk
• Place 15 - 26 mm apart
• Should see round zone of inhibition, but if zone for
clindamycin is blunted (capital D)…
– Isolate contains inducible erm gene
– Report: Erythromycin – R and Clindamycin – R
• If you see the expected round zone of inhibition for
clindamycin…
– Isolate has efflux-mediated erythromycin resistance (msrA gene)
– Report: Erythromycin – R and Clindamycin – S
• Detection of inducible clindamycin resistance now
incorporated into automated testing systems
Vancomycin-Resistant Enterococcus (VRE)
Enterococci naturally resistant to lots of antibiotics
– Cephalosporins, SXT, clindamycin
– Low level resistance to aminoglycosides (aminoglycoside modifying enzymes)
• Therapy for serious infections often requires combination therapy – cell wall-active agent + aminoglycoside
• But some enterococci can modify their ribosome resulting in…
High level aminoglycoside resistance: Detected by testing the isolate in 500 ug/mL gentamicin or 1000 ug/mL streptomycin
– If susceptible – combination therapy with cell wall agent (if susceptible) WILL BE effective
– If resistant – combination therapy with cell wall agent (if susceptible) WILL NOT BE effective
• And now there’s vancomycin resistance too
• VRE – nosocomial transmission
– Van A (E. faecium [most VRE isolates] and E. faecalis); High level R to Vanc (> 64 μg/ml)
– Van B (E. faecium and E. faecalis); Low level R to Vanc (4-32 μg/ml); S to teicoplanin
– Van C (E. gallinarum and E. casseliflavus)
• Low level R to Van (usually Vanc=I) and S to teicoplanin; Not nosocomial transmission
• Resistance mechanism – modified peptidoglycan precursor
Tx for VRE: linezolid and streoptogramins (quinupristin/dalfopristin)
Gram-neg bacilli
Extended-spectrum B-lactamase (ESBL)
• Point mutations of common (TEM and SHV) B-lactamases responsible for ampicillin resistance in E. coli, Klebsiella and Proteus
• The bad thing…
– Capable of hydrolyzing extended spectrum cephalosporins
– Outcome data suggests that they do cause treatment failures in vivo
• The good thing…
– They are inhibited by clavulanic acid
• DISK: ≥ 5mm zone increase = ESBL
• Confirmed ESBL producers should be reported as resistant to ALL penicillins and cephalosporins (regardless of in vitro result)
• Exceptions:
– Cephamycins – not hydrolyzed by ESBLs (Report as tested)
• Cefoxitin, cefotetan, cefmetazole
– B-lactam/ B-lactamase inhibitor combinations
• The inhibitors inhibit the ESBLs
• Amixicillin-clavulanate, piperacillin-tazobactam
Carbapenemases
Assoc c outbreaks of multi-resistant Klebsiella
pneumoniae, other Enterobacteriaceae, Pseudomonas and Acinetobacter
• Most common – KPC (K. pneumoniae carbapenemase)
-- other carbapenemase, like metallo B lactamase (MBL) and the SME-1 in Serratia marcescens can also make a positive Modified Hodge Test, but are not frequently seen in the US
– Plasmid-borne – Confers resistance to all B-lactams
– Easily spread
– Difficult to detect – Low level expression: Ertapenem is most sensitive screening drug
• Screening – Look for elevated carbapenem MICs (may still be ‘S’) AND resistance to at least one 3rd generation cephalosporin
• Confirmation – Modified Hodge test
• Drugs used to treat these isolates include tigecycline and colistin
Modified Hodge Test (MHT)
– Disk diffusion
– Isolate = QC organism (E. coli ATCC 25922)
– Disk = meropenem or ertapenem
– Patient isolate (spoke in the wheel): Streak from disk to outer edge of plate
– Incubate like disk diffusion
• Other important carbapenemases
– New Delhi metallo-B-lactamase (NDM-1) in E. coli, K. pneumoniae
– Verona Integron-Encoded metallo-B-lactamase (VIM) - K. pneumoniae and P. aeruginosa
– Imipenem-resistant metallo-beta-lactamase (IMP) – most often seen in P. aeruginosa and Acinetobacter
– Carbapenem-hydrolyzing class D beta-lactamases or oxacillinases (OXA) – do not have extended spectrum beta lactamase activity - Acinetobacter
Agar Diffusion Test

Example of MIC microtiter plate

Gradient diffusion Etest
E-test


MRSA Mechanism. Horizontally transferred DNA element - SCCmec; site specific recombination; mecA gene encodes PBP2a; PBP2a capable of cell wall synthesis; PBP2a has low affinity for all B-lactams

MRSA mech. 1. Modifying enzymes; 2 degrading enzymes; 3. target change; 4 efflux pumps
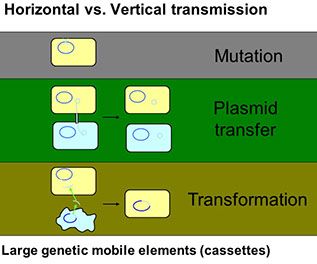

mecA is part of a large, mobile genetic element; Staphylococcal cassette chromosome mec (SCCmec)
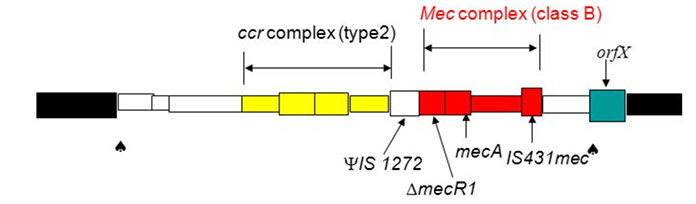
SCCmec cassette: a unique class of mobile genetic element (21-67 kb), resembles a pathogenicity island, but no virulence genes; ccr complex: ccrA and ccrB encode recombinase A and B enable SCCmec to integrate to chromosome in correct orientation; Mec complex: encodes B-lactam resistance and its inducible regulation +transposons and integrated copies of plasmids that carry various resistance genes (non-B-lactam). the mec complex: S aureus only has class A and B; class C mainly S haemolyticus, class D in S hominis
Inducible Clindamycin Resistance.
0 = Null Phenotype; c = constitutive phenotype, i = inducible phenotype.
-- Inducible strain originally reported as sensitive, but quickly becomes resistant

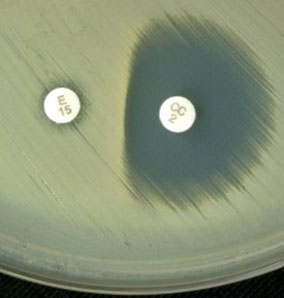
Inducible Clindamycin Resistance in S aureus. For D-test pos MRSA strains, CLSI recommends reporting them as "resistant" to clindamycin, as well as adding a susceptibility report

D-test pos. E = 15 ug Erythromycin disk, CC = 2 ug Clindamycin disk

Mechanism of vancomycin resistance. VanS is a membrane-bound histidine kinase that senses the presence of vancomycin,casuing ATP- dependent autophosphorylation. The phospho-VanS then transfers phosphate to the response regulator VanR in the cytoplasm. Phospho-VanR binds to the intergenic region upstream of the vanHAX operon and facilitates transcription, resulting in drug resistance. Among the resistance cassette proteins, VanX is a dipeptidase that removes D -Ala- D -Ala that continues to be generated
VRE Mechanism of Resistance

MHT: "Clover leaf" positivity in streaks 1 and 4. The samples on 1 and 4 contain a bacteria that produces a carbapenemase which allows the breakdown of carbapenem, which is diffusing from the central disk, streaks 2 and 3 have bacteria which are killed by carbapenem, and thus have no indentation around them

Antimycobacterials
M. tuberculosis
Prophylaxis: Isoniazid
Tx: Rifampin, Isoniazid, Pyrazinamide, Ethambutol ***RIPE***
M. avium intracellulare
Prophylaxis: Azithromycin
Tx: Azithromycin, rifampin, ethambutol, streptomycin
M. leprae
Prophylaxis: N/A
Tx: Dapsone, rifampin, clofazimine
Isoniazid (INH)
Mech: Dec synth of mycolic acids. Bacterial catalase peroxidase ((KatG) necessary to convert INH to active metabolite
Use: M tuberculosis - delays resistance to dapsone when used for leprosy;
- used for meningococcal prophylaxis and chemoprophylaxis in contacts of kiddos c H influenzae type B
Tox: Minocr hepatotoxicity and drug interactions (inc P-450); orange body fluids (non-hazardous side effect)
Pyrazinamide
Mech: Inhibits mycolic acid production by blocking mycobacterial fatty acid synth
- is effective in acidic pH of phagolysosomes where TB engulfed by macrophages are found
Tox: hyperuricemia and hepatotoxicity
Ethambutol
Mech: dec carbohydrate polymerization of mycobacterium cell wall by blocking arabinosyltransferase
Tox: Optic neuropathy (red-green color blindness)
Antimicrobial Prophylaxis
Meningococcal infx: Ciprofloxacin (drug of choice), rifampin, minocycline
Gonorrhea: Ceftriaxone
Syphilis: Benzathine penicillin G
Hx of recurrent UTIs: TMP-SMX
Endocarditis c surgical or dental procedure: Penicillins
HIV Prophylaxis
CD4<200
Prophylaxis: TMP-SMX
Infx: Pneumocystis pneumonia
CD4< 100
Prophylaxis: TMP-SMX
- may used aerosolized pntamide if pt cannot tolerate TMP-SMX, but this does not prevent toxoplasmosis infx concurrently
Infx: Pneumocystis pneumonia and toxoplasmosis
CD4 < 50
Prophylaxis: Azithromycin
Infx: Mycobacterium avium complex
Empiric tx for Community-Acquired Pneumonia
Outpt: Macrolides
Inpt: Fluoroquinolones
ICU setting: B-lactam + (fluoroquinolone OR azithromycin)
Antifungals
Membrane function: Amphoteracin B
Cell wall synth: Caspofungin and Micafungin
Nucleic acid synth: 5-fluorocytosine
Lanosterol synth: Naftifine, terbinafine
Ergosterol Synth: Fluconazole, Itraconazole, Voconozole
Amphotericin B
Mech: Binds ergosterol (unique to fungi); forms membrane pores that allow leakage of electrolytes
Clinical use: Serious, systemic mycoses, such as Cryptococcus, Blastomycosis, Coccidiodes, Aspergillus, Histoplasmosis, Candida, Mucor, or intrathecal for fungal meningitis
- does not cross blood-brain barrier
- supplement K and Mg bc of altered renal tubule permeability
Tox: fever/chills ("shake and bake") hypotension, nephrotox, arrhythmias, anemia, IV phlebitis ("amphoterrible")
- hydration reduces nephrotox
- liposomal amphotericin reduces tox
Nystatin
Mech: Same as amphotericin. Topical form bc too toxic for systemic use
Clinical use: "Swish and swallow" for oral candidiasis (thrush); topical for diaper rash or vaginal candidiasis
Fluconazole, ketoconazole, clotrimazole, microconazole, itraconazole, voriconazole (azoles)
Mech: inhibit fungal sterol (ergosterol) synth by inhibiting the P-450 enzyme that converts lanosterol to ergosterol
Clinical use: Systemic mycoses. Fluconazole for cryptococcal meningitis in AIDS pts (bc can cross BBB) and candidal infections of all types. Ketoconazole for Blastomyces, Coccidioides, Histoplasma, Candida albicans, hypercortisolism. Clotrimazole and miconazole for topical fungal infx
Tox: Hormone synth inhibition (gynecomastia), liver dysfunction (inhibits cytochrome P-450), fever, and chills
Flucytosine
Mech: Inhibits DNA synth by conversion to 5-fluorouracil by cytosine deaminase
Clinical use: Used in systemic fungal infx (eg Cryptococcus) in combination c amphoteracin B
Tox: Nausea, comiting, diarrhea, bone marrow suppression
Caspofungin
Mech: inhibits cell wall synth by inhibiting synth of B-glucan synth
Clinical use: Invasive aspergillosis, Candida
Tox: GI upset, flusing
Terbinafine
Mech: Inhibits fungal enzyme squalene epoxidase
Clinical use: dermatophytoses (esp onchomycosis - a fungal infx of the finger or toe nail)
Tox: abnormal LFTs, visual disturbances
Griseofulvin
Mech:interferes c microtubule function, disrupts mitosis, depends on keratin-containing tissues (eg nails)
Clinical use: oral tx of superficial infx, inhibits growth of dermatophytes (tinea, ringworms)
Tox: Teratogenic, carcinogenic, confusion, headaches, inc P450 and warfarin metabolism
Antiprotozoan thherapy
Pyrimethamine (toxoplasmosis or Plasmodium falciparum), suramin and melarsoprol (Trypanosoma brucei), nitrofurox (T cruzi), sodium stibogluconate (leishmaniasis)
Chloroquine
Mech: blocks plasmodium heme polymerase
Clinical use: Plasmodium spp. Also mefloquine (for treatment or prophylaxis)
- quinone for resistant spp in combo c pyrimethamine / sulfonamide
Tox: Retinopathy, G6PD hemolysis
Antihelminthic therapy
Mebendazole, pyrantel pamoate, ivermectin, diethylcarbamazine, praziquantelm immobilize helminths
Antivirals
Amantadine
Mech: blocks viral penetration / uncoating (M2 protein). Also causes release of dopamine for intract nerve terminals
Clinical use: prophylaxis and tx for influenza A only; also Parkinson dz (extrapyramidal sx)
Tox: Ataxia, dizziness, slurred speech, livedo reticularis
Mech of resistance: Mutated M2 protein; >90% of all influenza A strains resistant to amantadine so not used
Zanamivir, Oseltamivir
Mech: inhibits influena neuraminidase, dec release of progeny virus
Clinical uses: Both influena A and B
Ribavirin
Mech: inhibits synthesis of guanine nucleotides by competitively inhibiting IMP dehydrogenase
Clinical use: RSV, chronic hepatitis C
Tox: hemolytic anemia, severe teratogen
Acyclovir
Mech: monophosphorylated by HSV/VZV thymidine kinase. Guanosine analog. Triphosphate formed by cellular enzymes. Preferentially inhibits viral DNA polymerase by chain termination
Clinical use: HSV, VZV, EBV. Used for HSV-induced mucocutaneous and genital lesions as well as for encephalitis. Prophylaxis in immunocompromised pts. No effect on latent forms of HSV and VZV. Valacyclovir, a prodrug of acyclovir, has better oral bioavailability
Tox: few
Mech of resistance: Lack of viral thymidine kinase
Ganciclovir
Mech: 5'-monophosphate formed by a CMV viral kinase. Guanosine analog. Triphosphate formed by cellular kinases. Preferentially inhibits viral DNA polymerase
Clinical use: CMV, esp in immunocompromised pts. Valganciclovir is prodrug of ganciclovir and has better oral bioavailability (also used in pts c advanced HIV)
Tox: Leukopenia, neutropenia, TBCpenia, renal tox. More toxic to host enzymes than acyclovir
Mech of Reistance: Mutated CMV DNA polymerase or lack of viral kinase
Foscarnet
Mech: Viral DNA polymerase inhibitor that binds to pyrophosphate-binding site of enzyme. Does not require activation by viral kinase
Clinical use: CMV retinitis in immunocompromised pts when ganciclovir fails, or acyclovir-resistant HSV
Tox: nephrotoxicity (electrolyte abnormalities)
Mech of resistance: Mutated DNA polymerase
Cidofovir
Mech: preferentially inhibits viral DNA polymerase. Does not require phosphorylation by viral kinase
Clinical use: CMV retinitis in immunocompromised pts; acyclovir-resistant HSV. Long T1/2
Tox: Nephrotoxicity (coadministered c probenecid)
HIV Therapy
Highly Active Antiretroviral Therapy (HAART)
Initiated when pts present c AIDS-defining illness, low CD4 counts (<500 cells / mm^3), or high viral load
- regimen is 3 drugs to prevent resistance:
-- 2 NRTIs and 1 NNRTI OR 1 protease inhibitor OR 1 integrase inhibitor
Lopinavir, Atazanavir, Darunavir, Fosamprenavir, Saquinavir, Ritonavir (protease Inhibitors)
Mech: assembly of virions depends on HIV-1 protease (pol gene) that cleaves the polypeptide products of HIV mRNA into their functional parts; thus protease inhibitors prevent maturation of new viruses
- ritonavir can "boost" other drug concentrations by inhibiting P-450
- All protease inhibitors end in -navir
*** NAVIR tease a proTEASE ***
Tox: Hyperglycemia
Tenofovir (TDF), Emtricitabine (FTC), Abacavir (ABC), Lamivudine (3TC), Zidovudine (ZDV, formerly AZT), Didanosine (ddI), Stavudine (d4T), Nucleoside Reverse Transcriptiase Inhibitors (NRTIs)
Mech: Competitively inhibit nucleotide binding to reverse transcriptase and terminate the DNA chain (lack a 3'-OH group)
- must be phosphorylated by thymidine kinase to be active
- ZDV used for general prophylaxis and during pregnancy to reduce risk of fetal transmission
*** Have you dined (vudine) with my nuclear (nucleoside) family? ***
Tox: Bone marrow suppression (can be reversed c G-CSF and erythropoietin), peripheral neuropathy, lactic acidosis (nucleosides), rash (non-nucleosides), megaloblastic anemia (ZDV), pancreatitis (didanosin)
Nevirapine, Efavirenz, Delavirdine, Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs)
Bind to reverse transcriptase at site different from NRTIs. Do not require phosphorylation to be active or compete c nucleotides
*** Never, Ever, Deliver Nucleosides ***
Tox: Same as NRTIs
Raltegravir, Integrase Inhibitor
Mech: Inhibits HIV genome integration to host cell chromosome by reversibly inhibiting HIV integrase
Tox: Hypercholesterolemia
Interferons
Mech: Glycoproteins synthesized by virus-infected cells block replication of both RNA and DNA viruses
Clinical use: INF-a for chronic hepatitis B and C, Kaposi sarcoma
- INF-B for MS
- INF-gamma - NADPH oxidase deficiency
Tox: neutropenia
Antibiotics to Avoid in Pregnancy
Clarithromycin - embryotoxic
Sulfonamides - kernicterus
Aminoglycosides - ototoxic
Fluoroquinolones - cartilage damage
Metronidazole - mutagenesis
Tetracyclines - discolored teeth, inhibits bone growth
Ribavirin (antiviral) - teratogenic
Griseofulvin (antifungal) - teratogen
Chloramphenicol - "gray baby"
***Countless SAFe Moms Take Really Good Care ***
Referenctes
1. First Aid 2010 and 2012
2. Osler notes 2018
